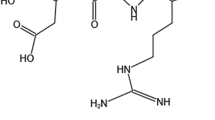
Abstract
Purpose
Several individual approaches were combined to fabricate a novel nanoparticulate drug delivery system to achieve targeting and anticancer effects in various malignant cancer cells.
Methods
Doxorubicin was conjugated to Poly(lactic-co-glycolic acid) (PLGA), which was formulated into nanoparticle via solvent-diffusion method. The surface of the nanoparticles was subsequently linked with Poly(ethylene glycol) (PEG) and Arg-Gly-Asp (RGD) peptide to realize both passive and active targeting functions. The multifunctional nanoparticles were then tested against several malignant cancer cell lines.
Results
The conjugation increased loading efficiency of doxorubicin to PLGA nanoparticles (the encapsulation efficiency was over 85%) and alleviated the drug burst release effect substantially. The drug was released from the polymeric matrix in a sustained release manner over a period of 12 days. The resultant nanoparticles were spherically uniform and well-dispersed. The nanoparticle targeting ability was proven through strong affinity to various integrin-expressing cancer cells, and much less affinity to the low integrin expression cancer cells. The nanoparticles also showed high efficacy in inducing apoptosis in specific malignant cancer cell.
Conclusion
The developed multifunctional nanoparticles hold potential to treat malignant integrin-expressing cancers.








Similar content being viewed by others
References
R. O. Hynes. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687 (2002). doi:10.1016/S0092-8674(02)00971-6.
R. O. Hynes. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 69:11–25 (1992). doi:10.1016/0092-8674(92)90115-S.
R. M. Schiffelers, G. A. Koning, T. L. M. ten Hagen, M. Fens, A. J. Schraa, A. Janssen, R. J. Kok, G. Molema, and G. Storm. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release. 91:115–122 (2003). doi:10.1016/S0168-3659(03)00240-2.
R. M. Schiffelers, A. Ansari, J. Xu, Q. Zhou, Q. Q. Tang, G. Storm, G. Molema, P. Y. Lu, P. V. Scaria, and M. C. Woodle. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 32:e149 (2004). doi:10.1093/nar/gnh140.
K. Temming, R. M. Schiffelers, G. Molema, and R. J. Kok. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updat. 8:381–402 (2005). doi:10.1016/j.drup.2005.10.002.
K. Temming, D. L. Meyer, R. Zabinski, E. C. Dijkers, K. Poelstra, G. Molema, and R. J. Kok. Evaluation of RGD-targeted albumin carriers for specific delivery of auristatin E to tumor blood vessels. Bioconjug Chem. 17:1385–1394 (2006). doi:10.1021/bc060087z.
P. M. Winter, S. D. Caruthers, A. Kassner, T. D. Harris, L. K. Chinen, J. S. Allen, E. K. Lacy, H. Zhang, J. D. Robertson, S. A. Wickline, and G. M. Lanza. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 63:5838–5843 (2003).
R. Langer. Drug delivery. Drugs on target. Science. 293:58–59 (2001). doi:10.1126/science.1063273.
M. E. Davis, Z. Chen, and D. M. Shin. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Reviews Drug Discovery. 7:771–782 (2008). doi:10.1038/nrd2614.
T. Lammers, W. E. Hennink, and G. Storm. Tumour-targeted nanomedicines: principles and practice. Br. J. Cancer. 99:392–397 (2008). doi:10.1038/sj.bjc.6604483.
K. J. Cho, X. Wang, S. M. Nie, Z. Chen, and D. M. Shin. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 14:1310–1316 (2008). doi:10.1158/1078-0432.CCR-07-1441.
S. Dandamudiand, and R. B. Campbell. The drug loading, cytotoxicty and tumor vascular targeting characteristics of magnetite in magnetic drug targeting. Biomaterials. 28:4673–83 (2007). doi:10.1016/j.biomaterials.2007.07.024.
G. S. Papaetis, C. Roussos, and K. N. Syrigos. Targeted therapies for non-small cell lung cancer. Curr. Pharm. Des. 13:2810–2831 (2007). doi:10.2174/138161207781757079.
S. H. Kim, J. H. Jeong, K. W. Chun, and T. G. Park. Target-specific cellular uptake of PLGA nanoparticles coated with poly(L-lysine)-poly(ethylene glycol)-folate conjugate. Langmuir. 21:8852–8857 (2005). doi:10.1021/la0502084.
Y. Bae, N. Nishiyama, and K. Kataoka. In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjug. Chem. 18:1131–1139 (2007). doi:10.1021/bc060401p.
D. Peer, J. M. Karp, S. Hong, O. C. FaroKhzad, R. Margalit, and R. Langer. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2:751–760 (2007). doi:10.1038/nnano.2007.387.
H. S. Yoo, K. H. Lee, J. E. Oh, and T. G. Park. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. J. Control. Release. 68:419–31 (2000). doi:10.1016/S0168-3659(00)00280-7.
J. E. Oh, Y. S. Nam, K. H. Lee, and T. G. Park. Conjugation of drug to poly(D,L-lactic-co-glycolic acid) for controlled release from biodegradable microspheres. J. Control. Release. 57:269–280 (1999). doi:10.1016/S0168-3659(98)00123-0.
H. S. Yoo, J. E. Oh, K. H. Lee, and T. G. Park. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm. Res. 16:1114–8 (1999). doi:10.1023/A:1018908421434.
N. Zhang, C. Chittasupho, C. Duangrat, T. J. Siahaan, and C. Berkland. PLGA nanoparticle–peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug. Chem. 19:145–152 (2008). doi:10.1021/bc700227z.
F. Chen, Y. Liu, J. Lu, K. J. Hwang, and V. H. Lee. A sensitive fluorometric assay for reducing sugars. Life Sci. 50:651–659 (1992). doi:10.1016/0024-3205(92)90450-4.
M. Herrmann, H. M. Lorenz, R. Voll, M. Grunke, W. Woith, and J. R. Kalden. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 22:5506–5507 (1994). doi:10.1093/nar/22.24.5506.
M. E. Keegan, S. M. Royce, T. Fahmy, and W. M. Saltzman. In vitro evaluation of biodegradable microspheres with surface-bound ligands. J. Control. Release. 110:574–580 (2006). doi:10.1016/j.jconrel.2005.11.004.
L. Vellon, J. A. Menendez, H. Liu, and R. Lupu. Up-regulation of alphavbeta3 integrin expression is a novel molecular response to chemotherapy-induced cell damage in a heregulin-dependent manner. Differentiation. 75:819–830 (2007). doi:10.1111/j.1432-0436.2007.00241.x.
O. H. Ramos, A. Kauskot, M. R. Cominetti, I. Bechyne, C. L. Salla Pontes, F. Chareyre, J. Manent, R. Vassy, M. Giovannini, C. Legrand, H. S. Selistre-de-Araujo, M. Crepin, and A. Bonnefoy. A novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clin. Exp. Metastasis. 25:53–64 (2008). doi:10.1007/s10585-007-9101-y.
B. R. Line, A. Mitra, A. Nan, and H. Ghandehari. Targeting tumor angiogenesis: Ccomparison of peptide and polymer-peptide conjugates. J. Nucl. Med. 46:1552–1560 (2005).
R. S. Yang, C. H. Tang, W. J. Chuang, T. H. Huang, H. C. Peng, T. F. Huang, and W. M. Fu. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 45:661–669 (2005). doi:10.1016/j.toxicon.2005.01.013.
W. Cai, D. W. Shin, K. Chen, O. Gheysens, Q. Cao, S. X. Wang, S. S. Gambhir, and X. Chen. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 6:669–676 (2006). doi:10.1021/nl052405t.
C. B. Pattillo, F. Sari-Sarraf, R. Nallamothu, B. M. Moore, G. C. Wood, and M. F. Kiani. Targeting of the antivascular drug combretastatin to irradiated tumors results in tumor growth delay. Pharm. Res. 22:1117–1120 (2005). doi:10.1007/s11095-005-5646-0.
W. Arap, R. Pasqualini, and E. Ruoslahti. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 279:377–380 (1998). doi:10.1126/science.279.5349.377.
E. A. Murphy, B. K. Majeti, L. A. Barnes, M. Makale, S. M. Weis, K. Lutu-Fuga, W. Wrasidlo, and D. A. Cheresh. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc. Natl. Acad. Sci. U. S. A. 105:9343–9348 (2008). doi:10.1073/pnas.0803728105.
G. Minotti, P. Menna, E. Salvatorelli, G. Cairo, and L. Gianni. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56:185–229 (2004). doi:10.1124/pr.56.2.6.
F. Meyer-Losic, J. Quinonero, V. Dubois, B. Alluis, M. Dechambre, M. Michel, F. Cailler, A. M. Fernandez, A. Trouet, and J. Kearsey. Improved therapeutic efficacy of doxorubicin through conjugation with a novel peptide drug delivery technology (Vectocell). J. Med. Chem. 49:6908–6916 (2006). doi:10.1021/jm0606591.
B. Rihovaand, and K. Kubackova. Clinical implications of N-(2-hydroxypropyl)methacrylamide copolymers. Current Pharmaceutical Biotechnology. 4:311–322 (2003). doi:10.2174/1389201033489711.
T. Lammers, V. Subr, P. Peschke, P. Kuhnlein, W. E. Hennink, K. Ulbrich, F. Kiessling, M. Heilmann, J. Debus, P. E. Huber, and G. Storm. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br. J. Cancer. 99:900–910 (2008). doi:10.1038/sj.bjc.6604561.
T. Lammers, P. Peschke, R. Kuehnlein, V. Subr, K. Ulbrich, P. Huber, W. Hennink, and G. Storm. Effect of intratumoral injection on the biodistribution and the therapeutic potential of HPMA copolymer-based drug delivery systems. Neoplasia. 8:788–795 (2006). doi:10.1593/neo.06436.
R. Duncan. Polymer conjugates as anticancer nanomedicines. Nat. Rev., Cancer. 6:688–701 (2006). doi:10.1038/nrc1958.
P. A. Vasey, S. B. Kaye, R. Morrison, C. Twelves, P. Wilson, R. Duncan, A. H. Thomson, L. S. Murray, T. E. Hilditch, T. Murray, S. Burtles, D. Fraier, E. Frigerio, and J. Cassidy. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin. Cancer Res. 5:83–94 (1999).
C. Deng, H. Y. Tian, P. B. Zhang, J. Sun, X. S. Chen, and X. B. Jing. Synthesis and characterization of RGD peptide grafted poly(ethylene glycol)-b-poly(L-lactide)-b-poly(L-glutamic acid) triblock copolymer. Biomacromolecules. 7:590–596 (2006). doi:10.1021/bm050678c.
O. C. Farokhzad, J. J. Cheng, B. A. Teply, I. Sherifi, S. Jon, P. W. Kantoff, J. P. Richie, and R. Langer. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. U. S. A. 103:6315–6320 (2006). doi:10.1073/pnas.0601755103.
K. Avgoustakis. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: Preparation, properties and possible applications in drug delivery. Current Drug Delivery. 1:321–333 (2004). doi:10.2174/1567201043334605.
L. E. van Vlerken, T. K. Vyas, and M. M. Amiji. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 24:1405–1414 (2007). doi:10.1007/s11095-007-9284-6.
S. M. Ryan, G. Mantovani, X. X. Wang, D. M. Haddleton, and D. J. Brayden. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opinion on Drug Delivery. 5:371–383 (2008). doi:10.1517/17425247.5.4.371.
S. Sengupta, D. Eavarone, I. Capila, G. Zhao, N. Watson, T. Kiziltepe, and R. Sasisekharan. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 436:568–572 (2005). doi:10.1038/nature03794.
S. Strieth, M. E. Eichhorn, A. Sutter, A. Jonczyk, A. Berghaus, and M. Dellian. Antiangiogenic combination tumor therapy blocking alpha(v)-integrins and VEGF-receptor-2 increases therapeutic effects in vivo. Int. J. Cancer. 119:423–431 (2006). doi:10.1002/ijc.21838.
M. Kim, J. Liao, M. L. Dowling, K. R. Voong, S. E. Parker, S. Wang, W. S. El-Deiry, and G. D. Kao. TRAIL inactivates the mitotic checkpoint and potentiates death induced by microtubule-targeting agents in human cancer cells. Cancer Res. 68:3440–3449 (2008). doi:10.1158/0008-5472.CAN-08-0014.
Acknowledgment
This study was supported by Academic Research Funding, grant number R148-000-097-112. Z. Wang is grateful to National University of Singapore for the financial support to his graduate studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Chui, WK. & Ho, P.C. Design of a Multifunctional PLGA Nanoparticulate Drug Delivery System: Evaluation of its Physicochemical Properties and Anticancer Activity to Malignant Cancer Cells. Pharm Res 26, 1162–1171 (2009). https://doi.org/10.1007/s11095-009-9837-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9837-y