Abstract
Purpose
The heparin–paclitaxel conjugates using amino acid as linker (HD2), with low anticoagulant activity, the similar anticancer activity as paclitaxel, offer great potential for further investigation.
Methods
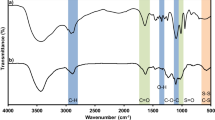
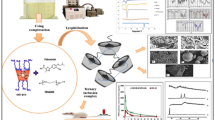
Two types of heparin–paclitaxel conjugates (HD) have been developed, in which O-acetylated heparin as carrier conjugates with paclitaxel by direct ester bond (HD1) and by inserting different amino acids as spacers, including valine, leucine, and phenylalanine (HD2a, HD2b, and HD2c), respectively. Specifically, mixed anhydride groups of carrier as activating intermediates mediate the synthesis of prodrugs. The HD conjugates are characterized by 1H NMR, FT-IR and GPC. The percentage weight of drug and hydrolysis rate for HD are detected by UV and HPLC. The anticoagulant activity and cell cycle of MCF-7 of HD are measured by APTT and FCM, respectively.
Results
HD2 conjugates show better solubility and faster hydrolysis rates than those of HD1. Meanwhile, the anticoagulant activity of HD is reduced and FCM analysis show that MCF-7 cells treated with HD are arrested in the G2/M phase of cell cycle.
Conclusions
Amino acids as linkers between paclitaxel and carrier are appropriate to facilitate the release of paclitaxel from carrier. Mixed anhydrides mediate the synthesis of prodrugs and HD2 conjugates are expected to further investigate in vivo experiment.











Similar content being viewed by others
References
R. B. Weiss, R. C. Donehower, P. H. Wiernik, T. Ohnuma, R. J. Gralla, D. L. Trump, J. R. Baker, D. A. Vanecho, D. D. Vonhoff, and B. Leyland-Jones. Hypersensitivity reactions from taxol. J. Clin. Oncol. 8:1263–1268 (1990).
H. M. Deutsch, J. A. Glinski, R. D. Haugwitz, V. L. Narayanan, M. Suffness, and L. H. Zalkow. Synthesis of congeners and prodrugs. 3. Water-soluble prodrugs of paclitaxel with potent antitumor activity. J. Med. Chem. 32:788–792 (1989). doi:10.1021/jm00124a011.
A. E. Mathew, M. R. Mejillano, J. P. Nath, R. H. Himes, and V. J. Stella. Synthesis and evaluation of some water-soluble prodrugs and derivatives of paclitaxel with antitumor activity. J. Med. Chem. 35:145–151 (1992). doi:10.1021/jm00079a019.
Z. Zhao, and D. G. I. Kingston. Modified taxols 6. Preparation of water-soluble prodrugs of taxol. J. Nat. Prod. 54:1607–1611 (1991). doi:10.1021/np50078a018.
Y. Ueda, A. B. Mikkilineni, J. O. Knipe, W. C. Rose, A. M. Casazza, and D. M. Vyas. Novel, water soluble phosphate prodrugs of taxol possessing in vivo antitumor activity. Bioorg. Med. Chem. Lett. 3:1761–1766 (1993). doi:10.1016/S0960-894X(00)80058-X.
M. V. Francesco, S. Oddone, P. Gianfranco, M. Raniero, and D. Ruth. PEG-doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjugate Chem. 16:775–784 (2005). doi:10.1021/bc040241m.
L. Hsiangfa, C. Sungching, C. Meichin, L. Powei, C. Chiungtong, and S. Hsingwen. Paclitaxel-loaded poly(γ-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system against cultured HepG2 cells. Bioconjugate Chem. 17:291–299 (2006). doi:10.1021/bc0502107.
S. Shu-ichi, K. Masahiro, K. Hiroshi, and K. To-ru. Complete regression of xenografted human carcinomas by a paclitaxel–carboxymethyl dextran conjugate (AZ10992). J. Controlled Release. 117:40–50 (2007). doi:10.1016/j.jconrel.2006.10.009.
F. M. H. De-Groot, C. Albrecht, R. Koekkoek, P. H. Beusker, and H. W. Scheeren. “Cascade-release dendrimers” liberate all end groups upon a single triggering event in the dendritic core. Angew. Chem. Int. Ed. 42:4490–4494 (2003). doi:10.1002/anie.200351942.
J. L. Robert. Heparin: Structure and Activity. J. Med. Chem. 46:2551–2563 (2003). doi:10.1021/jm030183a.
B. Casu, M. Guerrini, S. Guglieri, A. Naggi, M. Perez, G. Torri, G. Cassinelli, D. Ribatti, P. Carminati, G. Giannini, S. Penco, C. Pisano, M. Belleri, M. Rusnati, and M. Presta. Undersulfated and glycol-split heparins endowed with antiangiogenic activity. J. Med. Chem. 47:838–848 (2004). doi:10.1021/jm030893g.
I. Seiji, N. Hayao, H. Takashi, K. Tomoyuki, K. Yasuhiro, F. Zhang, K. Yasushi, I. Katsuki, A. Seiji, N. Akimasa, and T. Masae. Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a lightcycler. Cancer Lett. 183:195–203 (2002). doi:10.1016/S0304-3835(02)00157-X.
B. G. Richard, Z. Hong, and R. Prasanna. Synthesis, isolation, and characterization of 2’-paclitaxel glycinate: an application of the bsmoc protecting group. J. Org. Chem. 68:4894–4896 (2003). doi:10.1021/jo034077s.
S. Shu-ichi, K. Masahiro, K. Hiroshi, and K. To-ru. Paclitaxel delivery systems: the use of amino acid linkers in the conjugation of paclitaxel with carboxymethyl dextran to create prodrugs. Biol. Pharm. Bull. 25:632–641 (2002). doi:10.1248/bpb.25.632.
C. Peniche, W. Arguelles-Monal, N. Davidenko, R. Sastre, A. Gallardo, and J. Roman. Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation. Biomaterials. 20:1869–1878 (1999). doi:10.1016/S0142-9612(99)00048-4.
L. Chun, Y. Dong-fang, A. N. Robert, C. L. Fernando, S. Clifton, H. Nancy, M. Luka, and W. Sidney. Complete regression of well-established tumors using a novel water-soluble poly(l-glutamic acid)–paclitaxel conjugate. Cancer Res. 58:2404–2409 (1998).
Y. Yasuo, N. Hayao, K. Yuri, M. Seiichi, O. Kayoko, T. Masae, Y. Ikuo, and O. Minoru. Inhibition of experimental lung metastases of Lewis lung carcinoma cells by chemically modified heparin with reduced anticoagulant activity. Cancer Lett. 207:165–174 (2004). doi:10.1016/j.canlet.2003.11.037.
L. France, H. Kevin, L. Lun, J. T. Robert, S. Neil, W. Jennifer, J. S. Robert, C. John, and J. T. David. Chemical modifications of heparin that diminish its anticoagulant but preserve its heparanase-inhibitory, angiostatic, anti-tumor and anti-metastatic properties. Glycobiology. 6:355–366 (1996). doi:10.1093/glycob/6.3.355.
C. Benito, and N. Annamaria. Antiangiogenic heparin-derived heparan sulfate mimics. Pure. Appl. Chem. 75:157–166 (2003). doi:10.1351/pac200375020157.
K. Ono, M. Ishihara, K. Ishikawa, Y. Ozeki, H. Deguchi, M. Sato, H. Hashimoto, Y. Saito, H. Yura, A. Kurita, and T. Maehara. Periodate-treated, non-anticoagulant heparin -carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung. Br. J. Cancer. 86:1803–1812 (2002). doi:10.1038/sj.bjc.6600307.
P. B. Schiff, and S. B. Horwitz. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. U. S. A. 77:1561–1565 (1980). doi:10.1073/pnas.77.3.1561.
M. A. Jordan, K. Wendll, S. Gardiner, W. B. Derry, H. Copp, and L. Wilson. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56:816–825 (1996).
S. Y. Hyuk, and G. P. Tae. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J. Controlled Release. 96:273–283 (2004). doi:10.1016/j.jconrel.2004.02.003.
K. H. Bae, Y. Lee, and T. G. Park. Oil-encapsulating PEO-PPO-PEG/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules. 8:650–656 (2007). doi:10.1021/bm0608939.
ACKNOWLEDGEMENT
We thank Dr. Chunyi Tong from the Institute of Life Science and Biotechnology in Hunan University for his assistance with the experimental procedures during biomedical assays. The authors are grateful for the financial support of 985 Project of Ministry of Education. This work was supported by the National Natural Science Foundation of China (grant no. 20472018), by the Natural Science Foundation of Hunan (key project no. 07JJ3019) and Doctoral Fund of Ministry of Education of China (grant no. 20060532022) and Department of Science and Technology Foundation of Changsha (grant no. K0802152-31).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the linked to the electronic supplementary material
ESM 1
(DOC 32 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Xin, D., Liu, K. et al. Heparin–Paclitaxel Conjugates Using Mixed Anhydride as Intermediate: Synthesis, Influence of Polymer Structure on Drug Release, Anticoagulant Activity and In Vitro Efficiency. Pharm Res 26, 785–793 (2009). https://doi.org/10.1007/s11095-008-9762-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9762-5