Abstract
Purpose
To evaluate the sensitivity requirement for LC-MS/MS as an analytical tool to support human microdosing study with sub-pharmacological dose, investigate proportionality of pharmacokinetics from the microdose to therapeutic human equivalent doses in rats and characterize circulating metabolites in rats administered with the microdose.
Materials and Methods
Five drugs of antipyrine, metoprolol, carbamazepine, digoxin and atenolol were administered orally to male Sprague–Dawley rats at 0.167, 1.67, 16.7, 167 and 1,670 μg/kg doses. Plasma samples were extracted using either solid phase extraction or liquid–liquid extraction, and analyzed using LC-MS/MS.
Results
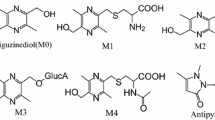
Using 100 μl of plasma sample, the lower limit of quantitation for antipyrine (10 pg/ml), carbamazepine (1 pg/ml), metoprolol (5 pg/ml), atenolol (20 pg/ml), and digoxin (5 pg/ml) were achieved using an API 5000™. Proportional pharmacokinetics were observed from 0.167 μg/kg to 1,670 μg/kg for antipyrine and carbamazepine and from 1.67 to 1,670 μg/kg for atenolol and digoxin, while metoprolol exhibited a non-proportional pharmacokinetics relationship. Several metabolites of carbamazepine were characterized in plasma from rats dosed at 1.67 μg/kg using LC-MS/MS.
Conclusions
This study has shown the promise of sensitive LC-MS/MS method to support microdose pharmacokinetics and drug metabolism studies in human.





Similar content being viewed by others
References
Food and Drug Administration. Guidance for industry, investigators, and reviewers—exploratory IND studies. January (2006).
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use (CHMP). 2004. Position paper on non-clinical safety studies to support clinical trials with a single microdose. CPMP, SWP, 2599, 02, Rev 1, London. 23 June (2004).
R. C. Garner, and G. Lappin. Commentary, the phase 0 microdosing concept. Br. J. Clin. Pharmacol. 61(4):367–370 (2006).
G. R. Zanni, and J. Y. Wick. Microdosing: the new pharmacokinetic paradigm? Consult Pharm. 21(10):756–776 (2006).
R. C. Garner. Less is more: the human microdosing concept. Drug Discov. Today 10(7):449–451 (2005).
G. Lappin, and R. C. Garner. The use of accelerator mass spectrometry to obtain early human ADME/PK data. Expert Opin. Drug Metab. Toxicol. 1(1):23–31 (2005).
G. Lappin, W. Kuhnz, R. Jochemsen, J. Kneer, A. Chaudhary, B. Oosterhuis, W. J. Drijfhout, M. Rowland, and R. C. Garner. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with five drugs. Clin. Pharmacol. Ther. 80(3):203–215 (2006).
P. Sandhu, J. S. Vogel, M. J. Rose, E. A. Ubick, J. E. Brunner, M. A. Wallace, J. K. Adelsberger, M. P. Baker, P. T. Henderson, P. G. Pearson, and T. A. Baillie. Evaluation of microdosing strategies for studies in preclinical drug development: demonstration of linear pharmacokinetics in dogs of a nucleoside analog over a 50-fold dose range. Drug Metab. Dispos. 32(11):1254–1259 (2004).
Food and Drug Administration. Safety Testing of Drug Metabolites. June (2005).
I. R. Wilding, and J. A. Bell. Improved early clinical development through human microdosing studies. DDT. 10(13):890–894 (2005).
S. K. Balani, N. V. Nagaraja, M. G. Qian, A. O. Costa, J. S. Daniels, H. Yang, P. R. Shimoga, J. T. Wu, L. S. Gan, F. W. Lee, and G. T. Miwa. Evaluation of Microdosing to assess pharmacokinetic linearity in rats using liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 34(3):384–388 (2006).
M. A. McLean, C. J. Tam, M. T. Baratta, C. L. Holliman, R. M. Ings, and G. R. Galluppi. Accelerating drug development: methodology to support first-in-man pharmacokinetics studies by the use of drug candidate microdosing. Drug Dev. Res. 68:14–22 (2007).
C. Y. Wu, and L. Z. Benet. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 22(1):11–23 (2005).
B. K. Park. Prediction of metabolic drug interactions involving beta-adrenoceptor blocking drugs. Br. J. Clin Pharmacol. 17(Suppl 1):3S–10S (1984).
D. G. McDevitt. Comparison of pharmacokinetic properties of beta-adrenoceptor blocking drugs. Eur. Heart J. 8(Suppl M):9–14 (1987).
L. Bertilsson, and T. Tomson. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide. An update. Clin. Pharmacokinet. 11(3):177–198 (1986).
Y. Tanigawara. Role of P-glycoprotein in drug disposition. Ther. Drug Monit. 22(1):137–140 (2000).
S. A. Coolen, T. Ligor, M. van Lieshout, and F. A. Huf. Determination of phenolic derivatives of antipyrine in plasma with solid-phase extraction and high-performance liquid chromatography-atmospheric-pressure chemical ionization mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 732(1):103–113 (1999).
Y. Zhu, H. Chiang, M. Wulster-Radcliffe, R. Hilt, P. Wong, C. B. Kissinger, and P. T. Kissinger. Liquid chromatography/tandem mass spectrometry for the determination of carbamazepine and its main metabolite in rat phasma utilizing an automated blood sampling system. J. Pharm. Biomed. Anal. 38(1):119–125 (2005).
N. Sarapa, P. H. Hsyu, G. Lappin, and R. C. Garner. The application of accelerator mass spectrometry to absolute bioavailability studies in humans: simultaneous administration of an intravenous microdose of 14C-nelfinavir mesylate solution and oral nelfinavir to healthy volunteers. J. Clin. Pharmacol. 45(10):1198–1205 (2005).
K. Lertratanangkoon, and M. G. Horning. Metabolism of carbamazepine. Drug Metab. Dispos. 10(1):1–10 (1982).
P. Myllynen, P. Pienimaki, H. Raunio, and K. Vahakangas. Microsomal metabolism of carbamazepine and oxcarbazepine in liver and placenta. Hum. Exp. Toxicol. 17(12):668–676 (1998).
Acknowledgments
The authors wish to thank Mr. Van Dinh and his group at Allergan for providing animal dosing and blood sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ni, J., Ouyang, H., Aiello, M. et al. Microdosing Assessment to Evaluate Pharmacokinetics and Drug Metabolism in Rats Using Liquid Chromatography-Tandem Mass Spectrometry. Pharm Res 25, 1572–1582 (2008). https://doi.org/10.1007/s11095-008-9555-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9555-x