Abstract
Purpose
To investigate the anomalous phenomenon of particle size shift during post-milling storage.
Materials and Methods
Crystallised and ball-milled adipic acid were stored under different humidity conditions. Analyses were carried out to characterise changes in particle size distribution (laser diffraction), morphology (SEM), bulk flow properties (annular shear tester), surface adhesion forces (AFM) and crystallinity (PXRD and DVS).
Results
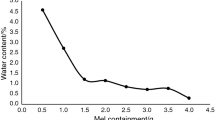
It was observed that the particle size distribution of milled adipic acid can shift to finer fractions, remain unchanged, or even shift to coarser fractions depending on storage conditions. SEM analysis showed that milled adipic acid is composed of agglomerates, which can undergo de-aggregation or further agglomeration via re-crystallisation. Empirical analysis ruled out the effects of electrostatic charges on the particle size shift. In addition, an improvement in powder flow in terms of bulk tensile strength was seen for milled adipic acid stored under high relative humidity but not under low humidity.
Conclusions
Storage of milled adipic acid below the critical relative humidity led to localised disintegration from the agglomerate surface and particle size reduction, which was not influenced by moisture sorption or loss. This evidence supports that “stress relaxation” mechanism behind particle breakage of post-milled particles. Appropriate storage conditions are important in maintaining the stability of milled powders.








Similar content being viewed by others
References
V. Chikhalia, R. T. Forbes, R. A. Storey, and M. Ticehurst. The effect of crystal morphology and mill type on milling induced crystal disorder. Eur. J. Pharm. Sci. 27:19–26 (2006).
M. D. Ticehurst, P. A. Basford, C. I. Dallman, T. M. Lukas, P. V. Marshall, G. Nichols, and D. Smith. Characterisation of the influence of micronisation on the crystallinity and physical stability of revatropate hydrobromide. Int. J. Pharm. 193:247–259 (2000).
G. H. Ward, and R. K. Shultz. Process-induced crystallinity changes in albuterol sulfate and its effect on powder physical stability. Pharm. Res. 12:773–779 (1995).
K. B. Pfeiffer, H. Haeusler, P. Grass, and P. Langguth. Influence on particle growth of salbutamol sulfate. Drug Dev. Ind. Pharm. 29:1077–1084 (2003).
K. B. Pfeiffer, P. Langguth, P. Grass, and H. Haeusler. Influence of mechanical activation on the physical stability of salbutamol sulphate. Eur. J. Pharm. Biopharm. 56:393–400 (2003).
V. Joshi, D. Sarvajna, and G. H. Ward. Increase in the specific surface area of budesonide during storage postmicronization. Pharm. Res. 19:7–12 (2002).
L. Mackin, S. Sartnurak, I. Thomas, and S. Moore. The impact of low levels of amorphous material (<5%) on the blending characteristics of a direct compression formulation. Int. J. Pharm. 231:213–226 (2002).
L. Mackin, R. Zanon, J. M. Park, K. Foster, H. Opalenik, and M. Demonte. Quantification of low levels (<10%) of amorphous content in micronised active batches using dynamic vapour sorption and isothermal microcalorimetry. Int. J. Pharm. 231:227–236 (2002).
P. Begat, P. M. Young, S. Edge, J. S. Kaerger, and R. Price. The effect of mechanical processing on surface stability of pharmaceutical powders: visualization by atomic force microscopy. J. Pharm. Sci. 92:611–619 (2003).
J. Subero, and M. Ghadiri. Breakage patterns of agglomerates. Powder Technol. 120:232–243 (2001).
K. Y. Chow, J. Go, M. Mehdizadeh, and D. J. W. Grant. Modification of adipic acid crystals: influence of growth in the presence of fatty acid additives on crystal properties. Int. J. Pharm. 20:3–24 (1984).
G. Steele. Preformulation as an aid to product design in early drug development. In M. Gibson (ed.), Pharmaceutical Preformulation and Formulation: A Practical Guide from Candidate Drug Selection to Commercial Dosage Form, Interpharm/CRC, Boca Raton, 2004, pp. 175–289.
T. R. Keel, C. Thompson, M. C. Davies, S. J. B. Tendler, and C. J. Roberts. AFM studies of the crystallization and habit modification of an excipient material, adipic acid. Int. J. Pharm 280:185–198 (2004).
Organisation Internationale de Métrologie Légale (OIML). The scale of relative humidity of air certified against saturated salt solutions. OIML: R121 (1996).
M. Mathlouthi, and B. Roge. Water vapour sorption isotherms and the caking of food powders. Food Chem. 82:61–71 (2003).
W. Pietsch. Agglomeration Process: Phenomena, Technologies, Equipment. Wiley-VCH, Weinheim Germany, 2002, pp. 35–59.
M. Suzuki. Powder mechanics. In K. Gotoh, H. Masuda, and K. Higashitani (eds.), Powder Technology Handbook, Marcel Dekker, New York, 1997, pp. 361–369.
R. Boerefin, Z. Ning, and M. Ghadiri. Disintegration of weak lactose agglomerates for inhalation applications. Int. J. Pharm. 172:199–209 (1998).
A. Samimi, R. Ghadiri, R. Boerefin, A. Groot, and R. Kohlus. Effect of structural characteristics on impact breakage of agglomerates. Powder Technol. 130:428–435 (2003).
K. Umprayn, and R. W. Mendes. Hygroscopicity and moisture adsorption-kinetics of pharmaceutical solids: a review. Drug Dev. Ind. Pharm. 13:653–693 (1987).
A. S. Gerhardt, C. Ahlneck, and G. Zografi. Assessment of disorder in crystalline solids. Int. J. Pharm. 101:237–247 (1994).
R. Huettenrauch, S. Fricke, and P. Zielke. Mechanical activation of pharmaceutical systems. Pharm. Res. 2:302–306 (1985).
T. E. Tarara, M. S. Hartman, H. Gill, A. A. Kennedy, and J. G. Weers. Characterization of suspension-based metered dose inhaler formulations composed of spray dried budesonide microcrystals dispersed in HFA-134a. Pharm. Res. 21:1607–1614 (2004).
J. R. Coury, and M. L. Aguiar. Rupture of dry agglomerates. Powder Technol 85:37–43 (1995).
R. Weichert. Anwendung von Fehlstellenstatistik und Bruchmechanik zur Bescreibung von Zerkleinerungsvorgaengen. Zement-Kalk-Gips 45:1–8 (1992).
F. M. Thomson. Storage of particulate solids. In M. E. Fayed, and L. Otten (eds.), Handbook of Powder Science, Chapman & Hall, New York, 1997, pp. 416–427.
H. Pasley, P. Haloulos, and S. Ledig. Stickiness—a comparison of test methods and characterization parameters. Dry. Technol. 13:1587–1601 (1995).
J. Staniforth. Powder flow. In A. E. Aulton (ed.), Pharmaceutics: The Science of Dosage Form Design, Churchhill Livingstone, Spain, 2001, pp. 197–210.
R. Jones. From single particle AFM studies of adhesion and friction to bulk flow: forging the links. Granul. Matter 4:191–204 (2003).
Acknowledgements
The authors would like to thank Junwei, Chin Lee, Ron Lim, Ai Tee, Prashant, Lay Yong, Gary Liu and Wenyi for their contribution in particle characterisation and Aaron Yuen for assisting in theoretical calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ng, W.K., Kwek, J.W. & Tan, R.B.H. Anomalous Particle Size Shift During Post-milling Storage. Pharm Res 25, 1175–1185 (2008). https://doi.org/10.1007/s11095-007-9497-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9497-8