Abstract
Purpose
The aim of this study was to gain insight into the feasibility of enhancing the delivery of L-Dopa and dopamine to the brain by linking these neurotransmitters and L-Dopa ethyl ester to 2-phenyl-3-carboxymethyl-imidazopyridine compounds giving rise to the so-called Dopimid compounds.
Materials and Methods
A number of Dopimid compounds were synthesized and both stability and binding studies to dopaminergic and benzodiazepine receptors were performed. To evaluate whether Dopimid compounds are P-gp substrates, [3H]ritonavir uptake experiments and bi-directional transport studies on confluent MDCKII-MDR1 monolayers were carried out. The brain penetration properties of Dopimid compounds were estimated by the Clark’s computational model and evaluated by investigation of their transport across BBMECs monolayers. The dopamine levels following the intraperitoneal administration of the selected Dopimid compounds were measured in vivo by using brain microdialysis in rat.
Results
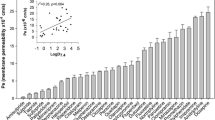
Tested compounds were adequately stable in solution buffered at pH 7.4 but undergo faster cleavage in dilute rat serum at 37°C. Receptor binding studies showed that Dopimid compounds are essentially devoid of affinity for dopaminergic and benzodiazepine receptors. [3H]ritonavir uptake experiments indicated that selected Dopimid compounds, like L-Dopa and dopamine hydrochloride, are not substrates of P-gp and it was also confirmed by bi-directional transport experiments across MDCKII-MDR1 monolayers. By Clark’s model a significant brain penetration was deduced for L-Dopa ethyl ester and dopamine derivatives. Transport studies involving BBMECs monolayers indicated that some of these compounds should be able to cross the BBB. Interestingly, the rank order of apparent permeability (P app) values observed in these assays parallels that calculated by the computational approach. Brain microdialysis experiments in rat showed that intraperitoneal acute administration of some Dopimid compounds induced a dose-dependent increase in cortical dopamine output.
Conclusion
Based on these results, it may be concluded that some Dopimid compounds can be proposed as novel L-Dopa and dopamine prodrugs.






Similar content being viewed by others
References
R. Bäckstrom, E. Honkanen, A. Pippuri, P. Kairisalo, J. Pystynen, K. Heinola, E. Nissinen, I. B. Linden, P. T. Männisto, S. Kaakkola, and P. Pohto. Synthesis of some novel potent and selective catechol O-methyltransferase inhibitors. J. Med. Chem. 32:841–846 (1989).
A. Di Stefano, B. Mosciatti, G. M. Cingolani, G. Giorgioni, M. Ricciutelli, I. Cacciatore, P. Sozio, and F. Claudi. Dimeric L-Dopa derivatives as potential prodrugs. Biorg. Med. Chem. Lett. 11:1085–1088 (2001).
H. Wang, J. Lee, M. Tsai, H. Lu, and W. Hsu. Synthesis and pharmcological activities of a novel tripeptide mimetic dopamine prodrug. Biorg. Med. Chem. Lett. 5:2195–2198 (1995).
R. Pahwa and W. C. Koller. Advances in the treatment of Parkinson’s disease. Drugs Today 34:95–105 (1998).
J. Jankovic and C. D. Marsden. Therapeutic strategies in Parkinson’s disease. In J. Jankovic and E. Tolosa (eds.), Parkinson’s Disease and Movement Disorders, Urban, Munich, 1988, pp. 95–119.
B. Asproni, A. Pau, M. Bitti, M. Melosu, R. Cerri, L. Dazzi, E. Seu, E. Maciocco, E. Sanna, F. Busonero, G. Talani, L. Pusceddu, C. Altomare, G. Trapani, and G. Biggio. Synthesis and pharmacological evaluation of 1-[(1,2-Diphenyl-1H-4-imidazolyl)methyl]-4-phenylpiperazines with clozapine-like mixed activities at dopamine D2, Serotonin and GABAA receptors. J. Med. Chem. 45:4655–4688 (2002).
J. Benavides, B. Peny, D. Ruano, J. Vitorica, and B. Scatton. Comparative autoradiographic distribution of central omega (benzodiazepine) modulatory site subtypes with high, intermediate and low affinity for zolpidem and alpidem. Brain Res. 604:240–250 (1993).
A. Durand, J. P. Thenot, G. Bianchetti, and P. L. Morselli. Comparative pharmacokinetic profile of two imidazopyridine drugs: zolpidem and alpidem. Drug Metab. Rev. 24:239–266 (1992).
A. Daniele, A. Albanese, G. Gainotti, B. Gregari, and P. Bartolomeo. Zolpidem in Parkinson’s disease. Lancet 349:1222–1223 (1997).
G. Trapani, V. Laquintana, A. Latrofa, J. Ma, K. Reed, M. Serra, G. Biggio, G. Liso, and J. M. Gallo. Peripheral benzodiazepine receptor ligand. Melphalan conjugates for potential selective drug delivery to brain tumors. Bioconjug. Chem. 14:830–839 (2003).
L. Nakonieczna, W. Przychodzen, and A. Chimiak. A new convenient route for the synthesis of DOPA peptides. Liebigs Ann. Chem. 1055–1058 (1994).
D. R. Cooper, C. Marrel, H. van de Waterbeemd, B. Testa, P. Jenner, and C. D. Marsden. L-Dopa esters as potential prodrugs: behavioural activity in experimental models of Parkinson’s disease. J. Pharm. Pharmacol. 39:627–635 (1987).
F. Tang, K. Horie, and R. T. Borchardt. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharm. Res. 19:765–772 (2002).
D. E. Clark. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 2. Prediction of blood–brain barrier penetration. J. Pharm. Sci. 88:815–821 (1999).
P. Ertl, B. Rohde, and P. Selzer. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 43:3714–3717 (2000).
K. L. Audus and R. T. Borchardt. Bovine brain microvessel endothelial cell monolayers as a model for blood–brain barrier. Ann. N.Y. Acad. Sci. USA 507:9–18 (1987).
D. Pal, K. L. Audus, and T. J. Siahaan. Modulation of cellular adhesión in bovine brain microvessel endotelial cells by a decapeptide. Brain Res. 747:103–113 (1997).
S. L. Glynn and M. Yazdanian. In vitro blood–brain barrier permeability of nevirapine compared to other HIV antiretroviral agents. J. Pharm. Sci. 87:306–310 (1998).
I. Megard, A. Garrigues, S. Orlowski, S. Jorajuria, P. Clayette, and E. Ezan, A. Mabondzo. A co-culture-based model of human blood–brain barrier: application to active transport of indinavir and in vivo–in vitro correlation. Brain Res. 927:153–157 (2002).
G. Paxinos and C. Watson. The rat brain in stereotaxic coordinates, Academic, London, 1982.
L. Dazzi, M. Serra, M. L. Porceddu, A. Sanna, M. F. Chessa, and G. Biggio. Enhancement of basal and pentylenetetrazol (PTZ)-stimulated dopamine release in the brain of freely moving rats by PTZ-induced kindling. Synapse 26:351–358 (1997).
M. D. Majewska, N. L. Harrison, R. D. Schwartz, J. L. Barker, and S. M. Paul. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007 (1986).
E. Giesen-Crouse. Peripheral benzodiazepine receptors, Academic Press, London, 1993.
G. Trapani, M. Franco, L. Ricciardi, A. Latrofa, G. Genchi, E. Sanna, F. Tuveri, E. Cagetti, G. Biggio, and G. Liso. Synthesis and binding affinity of 2-phenyl-imidazo[1,2-a]pyridine derivatives for both central and peripheral benzodiazepine receptors. A new series of high-affinity and selective ligands for the peripheral type. J. Med. Chem. 40:3109–3118 (1997).
G. Trapani, M. Franco, A. Latrofa, L. Ricciardi, A. Carotti, M. Serra, E. Sanna, G. Biggio, and G. Liso. Novel 2-phenyl-imidazo[1,2-a]pyridine derivatives as potent and selective ligands for peripheral benzodiazepine receptors. Synthesis, binding affinity, and in vivo studies. J. Med. Chem. 42:3934–3941 (1999).
G. Le Fur, M. L. Perrier, N. Vaucher, F. Imbant, A. Flamier, A. Uzan, C. Renault, M. C. Dubroeucq, and C. Gueremy. Peripheral binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 32:1839–1847 (1983).
A. Albert. Chemical aspects of selective toxicity. Nature 182:421–423 (1958).
A. Tsuji, T. Terasaki, Y. Takabatake, Y. Tenda, I. Tamai, T. Yamashima, S. Moritani, T. Tsuruo, and J. Yamashita. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 51:1427–1437 (1992).
M. Yamazaki, W. E. Neway, T. Ohe, I. Chen, J. F. Rowe, J. H. Hochman, M. Chiba, and J. H. Lin. In vitro substrate identification studies for P-glycoprotein-mediated transport: species difference and predictability of in vivo results. J. Pharmacol. Exp. Ther. 296:723–735 (2001).
N. Bodor and P. Buchwald. Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems. Adv. Drug Deliv. Rev. 36:229–254 (1999).
U. Norinder and M. Haeberlein. Computational approaches to the prediction of the blood–brain distribution. Adv. Drug Deliv. Rev. 54:291–313 (2002).
M. V. Shah, K. L. Audus, and R. T. Borchardt. The application of bovine brain microvessel endothelial-cell monolayers grown onto polycarbonate membranes in vitro to estimate the potential permeability of solutes through the blood–brain barrier. Pharm. Res. 7:624–627 (1989).
J. B. Jr Justice. Quantitative microdialysis of neurotransmitters. J. Neurosci. Methods 48:263–276 (1993).
A. Eltayb, M.-L. G. Wademberg, and T. H. Svensson. Enhanced cortical dopamine output and antipsycotic-like effect of raclopride with adjunctive low-dose L-Dopa. Biol. Psychiatry 58:337–343 (2005).
Acknowledgments
This work was supported by a grant from Ministero dell’Università e della Ricerca Scientifica e Tecnologica (MIUR) (COFIN 2003 of G.L.). We thank Mr. Giovanni Dipinto for skilful technical assistance in recording mass spectra. The authors would like to express their thanks to Dr. Soumyajit Majumdar from the Department of Pharmaceutics, School of Pharmacy, the University of Mississippi, for his helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Denora, N., Laquintana, V., Lopedota, A. et al. Novel L-Dopa and Dopamine Prodrugs Containing a 2-Phenyl-imidazopyridine Moiety. Pharm Res 24, 1309–1324 (2007). https://doi.org/10.1007/s11095-007-9255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9255-y