Abstract
Abstract
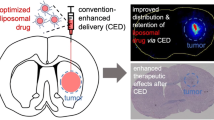
The surgical delivery of therapeutic agents into the parenchyma of the brain is problematic because it has been virtually impossible to know with any certainty where infused material is going, and how much to infuse. We have started to use liposomes loaded with Gadoteridol (GDL) as a tracer that allows us to follow infusions in real-time on magnetic resonance imaging (MRI). MRI allows precise tracking and measurement of liposomes loaded with markers and therapeutics. This review provides an overview of real-time delivery of liposomes to the central nervous system (CNS), and discusses the technical aspects of delivery, liposomes as colloidal systems of delivery, real-time distribution of liposomes in CNS, and quantification of liposome distribution. Our data suggests that real-time monitoring of liposomal drug infusion is likely to improve outcomes of clinical trials where convection-enhanced delivery (CED) is being used to target drugs to specific brain structures through limitation of systemic toxicity and reduction of side effects. This review is a summary of work done by our group over the past four years.













Similar content being viewed by others

References
C. H. Hsieh, Y. F. Chen, F. D. Chen, J. J. Hwang, J. C. Chen et al. Evaluation of pharmacokinetics of 4-borono-2-(18)F-fluoro-l-phenylalanine for boron neutron capture therapy in a glioma-bearing rat model with hyperosmolar blood–brain barrier disruption. J. Nucl. Med.46(11):1858–1865 (2005).
A. Weyerbrock, S. Walbridge, R. M. Pluta, J. E. Saavedra, L. K. Keefer et al. Selective opening of the blood–tumor barrier by a nitric oxide donor and long-term survival in rats with C6 gliomas. J. Neurosurg.99(4):728–737 (2003).
Y. Zhang, W. M. Pardridge et al. Delivery of beta-galactosidase to mouse brain via the blood–brain barrier transferrin receptor. J. Pharmacol. Exp. Ther.313(3):1075–1081 (2005).
R. H. Bobo, D. W. Laske, A. Akbasak, P. F. Morrison, R. L. Dedrick et al. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. U. S. A.91(6):2076–2080 (1994).
M. Y. Chen, R. R. Lonser, P. F. Morrison, L. S. Governale, and E. H. Oldfield. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J. Neurosurg.90(2):315–320 (1999).
D. W. Laske, P. F. Morrison, D. M. Lieberman, M. E. Corthesy, J. C. Reynolds et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J. Neurosurg.87(4):586–594 (1997).
P. F. Morrison, D. W. Laske, H. Bobo, E. H. Oldfield, and R. L. Dedrick. High-flow microinfusion: tissue penetration and pharmacodynamics. Am. J. Physiol.266(1 Pt 2):R292–R305 (1994).
S. Kunwar. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir., Suppl.88:105–111 (2003).
Z. Lidar, Y. Mardor, T. Jonas, R. Pfeffer, M. Faibel et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J. Neurosurg.100(3):472–479 (2004).
Y. Mardor, O. Rahav, Y. Zauberman, Z. Lidar, A. Ocherashvilli et al. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res.65(15): 6858–6863 (2005).
S. S. Gill, N. K. Patel, G. R. Hotton, K. O’Sullivan, R. McCarter et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med.9(5):589–595 (2003).
N. K. Patel, M. Bunnage, P. Plaha, C. N. Svendsen, P. Heywood et al. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann. Neurol.57(2):298–302 (2005).
A. A. Gabizon. Liposomal anthracyclines. Hematol./Oncol. Clin. North Am.8(2):431–450 (1994).
A. Gabizon, R. Isacson, E. Libson, B. Kaufman, B. Uziely et al. Clinical studies of liposome-encapsulated doxorubicin. Acta Oncol.33(7):779–786 (1994).
M. Zucchetti, A. Boiardi, A. Silvani, I. Parisi, S. Piccolrovazzi et al. Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother. Pharmacol.44(2):173–176 (1999).
J. T. Thigpen, C. A. Aghajanian, D. S. Alberts, S. M. Campos, A. N. Gordon et al. Role of pegylated liposomal doxorubicin in ovarian cancer. Gynecol. Oncol.96(1):10–18 (2005).
J. A. O’Shaughnessy. Pegylated liposomal doxorubicin in the treatment of breast cancer. Clin. Breast Cancer4(5):318–328 (2003).
K. J. Harrington, C. Lewanski, A. D. Northcote, J. Whittaker, A. M. Peters et al. Phase II study of pegylated liposomal doxorubicin (Caelyx) as induction chemotherapy for patients with squamous cell cancer of the head and neck. Eur. J. Cancer37(16):2015–2022 (2001).
S. R. Johnston and M. E. Gore. Caelyx: phase II studies in ovarian cancer. Eur. J. Cancer37(Suppl 9):S8–S14 (2001).
V. P. Torchilin. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov.4(2):145–160 (2005).
C. Mamot, J. B. Nguyen, M. Pourdehnad, P. Hadaczek, R. Saito et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J. Neuro oncol.68(1):1–9 (2004).
R. Saito, J. R. Bringas, T. R. McKnight, M. F. Wendland, C. Mamot et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res.64(7):2572–2579 (2004).
R. Saito, M. T. Krauze, J. R. Bringas, C. Noble, T. R. McKnight et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp. Neurol.196(2):381–389 (2005).
M. T. Krauze, T. R. McKnight, Y. Yamashita, J. Bringas, C. O. Noble et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res. Protoc.16(1–3):20–26 (2005).
P. F. Morrison, M. Y. Chen, R. S. Chadwick, R. R. Lonser, and E. H. Oldfield. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am. J. Physiol.277(4 Pt 2):R1218–R1229 (1999).
M. T. Krauze, R. Saito, C. Noble, M. Tamas, J. Bringas et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J. Neurosurg.103(5):923–929 (2005).
J. A. MacKay, D. F. Deen, and F. C. Szoka Jr. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res.1035(2):139–153 (2005).
R. Saito, M. T. Krauze, C. O. Noble, M. Tamas, and D. C. Drummond et al. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: Implications for local drug delivery. J. Neurosci. Methods154(1–2):225–232 (2006).
R. S. R. Yamashita, M. T. Krauze, C. O. Noble, T. Kawaguchi, and K. S. Bankiewicz. Convection-enhanced delivery of liposomal doxorubicin in intracranial brain tumor xenografts. Targeted-Oncology (2006) in press.
C. O. Noble, M. T. Krauze, D. C. Drummond, Y. Yamashita, R. Saito et al. Novel Nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: Pharmacology and efficacy. Cancer Res.66(5):2801–2806 (2006).
M. T. Krauze, R. Saito, C. Noble, J. Bringas, J. Forsayeth et al. Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp. Neurol.196(1):104–111 (2005).
P. Hadaczek, Y. Yamashita, M. Mirek, and K. S. Bankiewicz. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Molec. Ther.14(1):69–78 (2006).
J. F. Hamilton, P. F. Morrison, M. Y. Chen, J. Harvey-White, R. S. Pernaute et al. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp. Neurol.168(1):155–161 (2001).
Acknowledgments
Special thanks to BrainLAB for support and help with iPlan® software, and to Hermes Biosciences for the generous supply of liposomes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krauze, M.T., Forsayeth, J., Park, J.W. et al. Real-time Imaging and Quantification of Brain Delivery of Liposomes. Pharm Res 23, 2493–2504 (2006). https://doi.org/10.1007/s11095-006-9103-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9103-5