Purpose
Because of their importance in pharmaceutical applications, hydroxypropyl-β-cyclodextrin and methyl-β-cyclodextrin have been selected to study the formation of micronized complexes incorporating active pharmaceutical ingredients (APIs) and cyclodextrins (CDs) by dense gas (DG) processing.
Methods
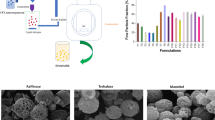
A single-step DG technique was used as an alternative to conventional methods for the manufacturing of API/CD complexes. The DG technology is highly attractive in the pharmaceutical industry because of its potential to generate micronized particles with controlled particle size distributions at moderate operating conditions. The effect of the aerosol solvent extraction system (ASES) processing on the dissolution performance of naproxen (NPX) was examined.
Results
The CDs were produced as microspheres smaller than 3 μm. The coprecipitation of each CD with NPX resulted in the production of microparticles with enhanced dissolution rates.
Conclusions
The ASES was operated under mild conditions and generated micron-sized spherical particles that could be of particular interest in formulations for pulmonary delivery.
Particular advantages of the technique are that (1) nontoxic solvents are used, and (2) it is suitable for the processing of thermally labile compounds. The proposed process can create opportunities to improve current administration routes and exploit novel delivery systems for drug formulations incorporating CDs.









Similar content being viewed by others
Abbreviations
- API:
-
active pharmaceutical ingredient
- ASES:
-
aerosol solvent extraction system
- CD:
-
cyclodextrin
- DELOS:
-
depressurization of an expanded liquid organic solution
- HP-β-CD:
-
hydroxypropyl-β-cyclodextrin
- M-β-CD:
-
methyl-β-cyclodextrin
- NPX:
-
naproxen
- PGSS:
-
particles from gas-saturated solutions
- SAA:
-
supercritical assisted atomization
References
J. Szejtli (1988) Cyclodextrin Technology Kluwer Academic Publishers Dordrecht, The Netherlands
T. Loftsson (2002) ArticleTitleCyclodextrins and the biopharmaceutics classification system of drugs J. Incl. Phenom. Macrocycl. Chem. 44 63–67 Occurrence Handle10.1023/A:1023088423667 Occurrence Handle1:CAS:528:DC%2BD3sXkvFCqtLY%3D
D. O. Thompson (1997) ArticleTitleCyclodextrins-enabling excipients: their present and future use in pharmaceuticals Crit. Rev. Ther. Drug Carr. Syst. 14 1–104 Occurrence Handle1:CAS:528:DyaK2sXhtlems7g%3D
J. Szejtli (1994) ArticleTitleMedicinal applications of cyclodextrins Med. Res. Rev. 14 353–386 Occurrence Handle1:CAS:528:DyaK2cXls1ekt7o%3D Occurrence Handle8007740
V. J. Stella R. A. Rajewski (1997) ArticleTitleCyclodextrins: their future in drug formulation and delivery Pharm. Res. 14 556–567 Occurrence Handle10.1023/A:1012136608249 Occurrence Handle1:CAS:528:DyaK2sXjtlSksrg%3D Occurrence Handle9165524
L. Szente J. Szejtli (1999) ArticleTitleHighly soluble cyclodextrin derivatives: chemistry, properties, and trends in development Adv. Drug Deliv. Rev. 36 17–28 Occurrence Handle10.1016/S0169-409X(98)00092-1 Occurrence Handle1:CAS:528:DyaK1MXksFegtw%3D%3D Occurrence Handle10837706
J. Szejtli T. Osa (1996) Cyclodextrins Elsevier Science Ltd. New York
R. A. Rajewski V. J. Stella (1996) ArticleTitlePharmaceutical applications of cyclodextrins. 2. In vivo drug delivery J. Pharmacol. Sci. 85 1143–1169
F. W. H. M. Merkus J. C. Verhoef E. Marttin S. G. Romeijn P. H. M. Kuy Particlevan der W. A. J. J. Hermens N. G. M. Schipper (1999) ArticleTitleCyclodextrins in nasal drug delivery Adv. Drug Deliv. Rev. 36 41–57 Occurrence Handle10.1016/S0169-409X(98)00054-4 Occurrence Handle1:CAS:528:DyaK1MXksFegtQ%3D%3D Occurrence Handle10837708
J. M. C. L. Pinto H. M. C. Marques (1999) ArticleTitleBeclomethasone/cyclodextrin inclusion complex for dry powder inhalation STP Pharma Sci. 9 253–256 Occurrence Handle1:CAS:528:DyaK1MXlsleiu7Y%3D
A. Clark, M. C. Kuo, and C. Lalor. Phospholipids, cyclodextrins, starch, and cellulose as hygroscopic growth inhibitors in dry powders for pulmonary drug delivery, Inhale Therapeutic Systems, Inc., USA, WO, 2000, pp. 46.
B. Cappello C. Maio ParticleDi M. Iervolino (2002) ArticleTitleInvestigation on the interaction of bendazac with β-, hydroxypropyl-β-, and γ-cyclodextrins J. Incl. Phenom. Macrocycl. Chem. 43 251–257 Occurrence Handle10.1023/A:1021282110659 Occurrence Handle1:CAS:528:DC%2BD3sXksVOgsA%3D%3D
Y. H. Chou and D. L. Tomasko. Gas crystallization of polymer–pharmaceutical composite particles. The 4th International Symposium on Supercritical Fluids, Vol. A, ISSF, Sendai, Japan, 1997, pp. 55–57.
G. Bettinetti A. Gazzaniga P. Mura F. Giordano M. Setti (1992) ArticleTitleThermal behavior and dissolution properties of naproxen in combinations with chemically modified β-cyclodextrins Drug Dev. Ind. Pharm. 18 39–53 Occurrence Handle1:CAS:528:DyaK38XhtFensbk%3D
P. Mura G. Bettinetti F. Melani A. Manderioli (1995) ArticleTitleInteraction between naproxen and chemically modified β-cyclodextrins in the liquid and solid state Eur. J. Pharm. Sci. 3 347–355 Occurrence Handle1:CAS:528:DyaK2MXpvVyqtbY%3D Occurrence Handle10.1016/0928-0987(95)00025-X
M. Charoenchaitrackool F. Dehgani N. R. Foster (2002) ArticleTitleUtilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-β-cyclodextrin Int. J. Pharm. 239 103–112
R. Thiering F. Dehghani A. Dillow N. R. Foster (2000) ArticleTitleSolvent effects on the controlled dense gas precipitation of model proteins J. Chem. Technol. Biotechnol. 75 42–53 Occurrence Handle1:CAS:528:DC%2BD3cXhtVSnsLg%3D
R. Thiering F. Dehghani A. Dillow N. R. Foster (2000) ArticleTitleThe influence of operating conditions on the dense gas precipitation of model proteins J. Chem. Technol. Biotechnol. 75 29–41 Occurrence Handle1:CAS:528:DC%2BD3cXhtVSnsLs%3D
G. S. Gurdial. Solubility behaviour of organic compounds in supercritical carbon dioxide, PhD dissertation, School of Chemical Engineering and Industrial Chemistry, University of New South Wales, Sydney, Australia, 1991.
E. Reverchon (1999) ArticleTitleSupercritical antisolvent precipitation of micro- and nano-particles J. Supercrit. Fluids 15 1–21 Occurrence Handle10.1016/S0896-8446(98)00129-6 Occurrence Handle1:CAS:528:DyaK1MXhsVSmu7k%3D
N. Ventosa S. Sala J. Veciana J. Torres J. Llibre (2001) ArticleTitleDepressurization of an expanded liquid organic solution (DELOS): a new procedure for obtaining submicron- or micron-sized crystalline particles Cryst. Growth Des. 1 299–303 Occurrence Handle10.1021/cg0155090 Occurrence Handle1:CAS:528:DC%2BD3MXkt1yru7Y%3D
E. Reverchon G. Porta ParticleDella A. Spada A. Antonacci (2004) ArticleTitleGriseofulvin micronization and dissolution rate improvement by supercritical assisted atomization J. Pharm. Pharmacol. 56 1379–1387 Occurrence Handle10.1211/0022357044751 Occurrence Handle1:CAS:528:DC%2BD2cXpvFOltLc%3D Occurrence Handle15525444
E. Reverchon G. Porta ParticleDella (2003) ArticleTitleMicronization of antibiotics by supercritical assisted atomization J. Supercrit. Fluids 26 243–252 Occurrence Handle1:CAS:528:DC%2BD3sXkvVehur0%3D
J. Fages H. Lochard J.-J. Letourneau M. Sauceau E. Rodier (2004) ArticleTitleParticle generation for pharmaceutical applications using supercritical fluid technology Powder Technol. 141 219–226 Occurrence Handle1:CAS:528:DC%2BD2cXks1Wiu7c%3D
X. Han, A. R. Baxter, K. W. Koelling, D. L. Tomasko, and L. J. Lee. Influences of solubility and viscosity in the polystyrene/CO2 microcellular foaming extrusion, 60th Annual Technical Conference—Society of Plastics Engineers, San Francisco, CA, 2002, pp. 1910–1914.
A. J. Busby, K. S. Morley, C. J. Roberts, M. S. Watson, P. B. Webb, B. Wong, J. Zhang, G. Kokturk, and S. M. Howdle. Polymers, biomaterials and supercritical fluids, Proceedings of the 8th Meeting on Supercritical Fluids, Vol. 1, Bordeaux, 2002, pp. 115–120.
F. Trotta M. Zanetti G. Camino (2000) ArticleTitleThermal degradation of cyclodextrins Polym. Degrad. Stab. 69 373–379 Occurrence Handle1:CAS:528:DC%2BD3cXlsl2iu7c%3D
J. Sztatisz, S. Gal, J. Komives, A. Stadler-Szoke, and J. Szejtli. Thermoanalytical investigations on cyclodextrin inclusion compounds, 1st International Symposium on Cyclodextrins, Budapest, 1981.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mammucari, R., Dehghani, F. & Foster, N.R. Dense Gas Processing of Micron-Sized Drug Formulations Incorporating Hydroxypropylated and Methylated Beta-Cyclodextrin. Pharm Res 23, 429–437 (2006). https://doi.org/10.1007/s11095-005-9094-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-9094-7