No Heading
Purpose.
To assess and compare the effectiveness of two types of polysaccharide-based micelles as delivery vehicles for poorly water soluble drugs by monitoring their permeability across Caco-2 cell monolayers.
Methods.
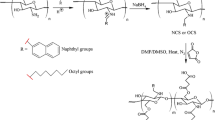
Dextran (DEX) and hydroxypropylcellulose (HPC) were hydrophobically modified (HM) by grafting polyoxyethylene cetyl ether (POE-C16, 15 mol% and 5.4 mol%, respectively). The onset of micellization and mean diameter of polymeric micelles formed by HM-DEX and HM-HPC were determined by fluorescence spectroscopy and dynamic light scattering, respectively. Cyclosporin A (CsA)-loaded polymeric micelles were prepared by a dialysis procedure, and the amount of incorporated CsA was assayed by high performance liquid chromatography (HPLC). The stability of micelles in simulated gastric and intestinal fluids was studied as a function of contact time, and their cytotoxicity toward Caco-2 cells was evaluated using the MTT colorimetric assay. The bidirectional transport across Caco-2 cell monolayers of CsA entrapped in HM-DEX and HM-HPC micelles and of the polymers themselves was evaluated in the presence and absence of P-glycoprotein inhibitor.
Results.
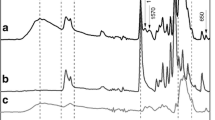
The amount of CsA incorporated in HM-HPC and HM-DEX micelles reached 5.5 and 8.5% w/w, respectively (entrapment efficiency of 22% or more). The polymeric micelles exhibited high stability in gastric and intestinal fluids and no significant cytotoxicity toward Caco-2 cells. The apical to basal permeability of CsA across Caco-2 cells increased significantly when loaded in polymeric micelles compared to free CsA.
Conclusions.
Polysaccharide-based polymeric micelles are promising carriers for the oral delivery of poorly water soluble drugs. In vitro tests indicate that, overall, HM-HPC micelles are more effective compared to HM-DEX micelles.
Similar content being viewed by others
Abbreviations
- AP:
-
apical side
- BL:
-
basolateral side
- CsA:
-
cyclosporin A
- DEX:
-
dextran T10
- HPC:
-
hydroxypropylcellulose
- P85:
-
Pluronic P85
- PGI:
-
P-glycoprotein inhibitor
- P-gp:
-
P-glycoprotein
- POE-C16:
-
polyoxyethylene (10) cetyl ether
References
1. R. M. Merion, D. J. White, S. Thiru, D. D. Evans, and R. Y. Calne. Cyclosporine: five years experience in cadaveric renal transplantation. N. Engl. J. Med. 310:148–154 (1984).
2. I. J. Klompmaker, J. M. Wierda, W. J. Sluiter, D. R. Uges, E. B. Haagsma, R. Verwer, and M. J. Slooff. Pharmacokinetics of cyclosporine A after intravenous and oral administration in liver transplant patients measured with high-performance liquid chromatography. Ther. Drug Monit. 15:60–64 (1993).
3. F. Galla, V. Marzocchi, L. Croattino, D. Poz, M. Baraldo, and M. Furlanut. Oral and intravenous disposition of cyclosporine in psoriatic patients. Ther. Drug Monit. 17:302–304 (1995).
4. A. C. Foradori, L. Martinez, A. Vacarezza, L. Elberg, A. Loveluck, and C. Pinto. Pharmacokinetics of a new galenical formulation of oral cyclosporine A in stable kidney transplanted patients. Transplant. Proc. 26:2969–2972 (1994).
5. I. S. Sketris, J. G. Lawen, L. Beauregard-Zollinger, P. Belitsky, D. Landsberg, M. L. Givner, and P. Keown. Comparison of the pharmacokinetics of cyclosporine sandimmune with sandimmune neoral in stable renal transplant patients. Transplant. Proc. 26: 2961–2963 (1994).
6. D. M. Woodcock, S. Jefferson, M. E. Linsenmeyer, P. J. Crowther, G. M. Chojnowski, B. Williams, and I. Bertoncello. Reversal of the multidrug resistance phenotype with cremophor EL, a common vehicle for water-insoluble vitamins and drugs. Cancer Res. 50:4199–4203 (1990).
7. F. Seeballuck, M. B. Ashford, and C. M. O’Driscoll. The effects of Pluronics block copolymers and Cremophor EL on intestinal lipoprotein processing and the potential link with P-glycoprotein in Caco-2 cells. Pharm. Res. 20:1085–1092 (2003).
8. J. Mason. Renal side-effects of cyclosporin A. Br. J. Dermatol. 122:71–77 (1990).
9. K. L. Skorecki, W. P. Rutledge, and R. W. Schrier. Acute cyclosporine nephrotoxicity-prototype for a renal membrane signalling disorder. Kidney Int. 42:1–10 (1992).
10. L. S. Friedman, J. L. Dienstag, P. W. Nelson, P. S. Russell, and A. B. Cosimi. Anaphylactic reaction and cardiopulmonary arrest following intravenous cyclosporine. Am. J. Med. 78:343–345 (1985).
11. D. L. Howrie, R. J. Ptachcinski, B. P. Griffith, R. J. Hardesty, J. T. Rosenthal, G. J. Burckart, and R. Venkataramanan. Anaphylactoid reactions associated with parenteral cyclosporine use: possible role of Cremophor EL. Drug Intell. Clin. Pharm. 19: 425–427 (1985).
12. C. Allen, D. Maysinger, and A. Eisenberg. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 16:3–27 (1999).
13. X. Chen, T. Young, M. Sarkari, R. Williams, and K. Johnston. Preparation of cyclosporine A nanoparticles by evaporative precipitation into aqueous solution. Int. J. Pharm. 242:3–14 (2002).
14. A. Sanchez and M. J. Alonso. Poly(D,L-lactide-co-glycolide) micro and nanospheres as a way to prolong blood/plasma levels of subcutaneously injected cyclosporin A. Eur. J. Pharm. Biopharm. 41:31–37 (1995).
15. K. Itoh, A. Pongpeerapat, Y. Tozuka, T. Oguchi, and K. Yamamoto. Nanoparticle formation of poorly water-soluble drugs from ternary ground mixtures with PVP and SDS. Chem. Pharm. Bull. (Tokyo) 51:171–174 (2003).
16. M. Al-Meshal, S. H. Khidr, M. A. Bayomi, and A. A. Al-Angary. Oral administration of liposomes containing cyclosporine: a pharmacokinetic study. Int. J. Pharm. 168:163–168 (1998).
17. B. Ozpolat, G. Lopez-Berestein, P. Adamson, C. J. Fu, and A. H. Williams. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J. Pharm. Pharm. Sci. 6:292–301 (2003).
18. F. Nacka, M. Cansell, P. Meleard, and N. Combe. Incorporation of alpha-tocopherol in marine lipid-based liposomes: in vitro and in vivo studies. Lipids 36:1313–1320 (2001).
19. M. C. Taira, N. S. Chiaramoni, K. M. Pecuch, and S. Alonso-Romanowski Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv. 11:123–128 (2004).
20. S. Bonduelle, M. Carrier, C. Pimienta, J. P. Benoit, and V. Lenaerts. Tissue concentration of nanoencapsulated radiolabelled cyclosporin following peroral delivery in mice or ophthalmic application in rabbits. Eur. J. Pharm. Biopharm. 42:313–319 (1996).
21. J. Molpeceres, M. R. Aberturas, and M. Guzman. Biodegradable nanoparticles as a delivery system for cyclosporine: preparation and characterization. J. Microencapsul. 17:599–614 (2000).
22. E. Ugazio, R. Cavalli, and M. R. Gasco. Incorporation of cyclosporin A in solid lipid nanoparticles (SLN). Int. J. Pharm. 241: 341–344 (2002).
23. J. Daia, T. Nagaib, X. Wanga, T. Zhangc, M. Menga, and Q. Zhang. pH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int. J. Pharm. 280:229–240 (2004).
24. M. P. Desai, V. Labhasetwar, G. L. Amidon, and R. J. Levy. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 13:1838–1845 (1996).
25. M. C. Varela, M. Guzman, J. Molpeceres, M. D. R. Aberturas, D. Rodriguez-Puyol, and M. Rodriguez-Puyol. Cyclosporine-loaded polycaprolactone nanoparticles: immunosuppression and nephrotoxicity in rats. Eur. J. Pharm. Sci. 12:471–478 (2001).
26. R. Gref, P. Quellec, A. Sanchez, P. Calvo, E. Dellacherie, and M. J. Alonso. Development and characterization of CyA-loaded poly(lactic acid)-poly(ethylene glycol)PEG micro- and nanoparticles. Comparison with conventional PLA particulate carriers. Eur. J. Pharm. Biopharm. 51:111–118 (2001).
27. A. M. De Campos, A. Sanchez, and M. J. Alonso. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 224:159–168 (2001).
28. G. Sertsou, J. Butler, J. Hempenstall, and T. Rades. Solvent change co-precipitation with hydroxypropyl methylcellulose phthalate to improve dissolution characteristics of a poorly water-soluble drug. J. Pharm. Pharmacol. 54:1041–1047 (2002).
29. M. F. Francis, L. Lavoie, F. M. Winnik, and J. C. Leroux. Solubilization of cyclosporin A in dextran-g-polyethyleneglycolalkyl ether polymeric micelles. Eur. J. Pharm. Biopharm. 56:337–346 (2003).
30. M. F. Francis, M. Piredda, and F. M. Winnik. Solubilization of poorly water soluble drugs in micelles of hydrophobically modified hydroxypropylcellulose copolymers. J. Control. Rel. 93: 59–68 (2003).
31. D. D. Lasic. Mixed micelles in drug delivery. Nature 355:279–280 (1992).
32. H. E. J. Hofland, J. A. Bouwstra, J. C. Verhoef, G. Buckton, B. Z. Chowdry, M. Ponec, and H. E. Junginger. Safety aspects of non-ionic surfactant vesicles-a toxicity study related to the physicochemical characteristics of non-ionic surfactants. J. Pharm. Pharmacol. 44:287–294 (1992).
33. B. G. Yu, T. Okano, K. Kataoka, S. Sardari, and G. S. Kwon. In vitro dissociation of antifungal efficacy and toxicity for amphotericin B-loaded poly(ethylene oxide)-block-poly(beta benzyl L-aspartate) micelles. J. Control. Rel. 56:285–291 (1998).
34. S. C. Kim, D. W. Kim, Y. H. Shim, J. S. Bang, H. S. Oh, S. W. Kim, and M. H. Seo. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J. Control. Rel. 72:191–202 (2001).
35. R. Jevprasesphant, J. Penny, D. Attwood, N. B. McKeown, and A. D’Emanuele. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm. Res. 20: 1543–1550 (2003).
36. C. Larsen. Dextran prodrugs-structure and stability in relation to therapeutic activity. Adv. Drug Deliv. Rev. 3:103–154 (1989).
37. N. P. Couch. The clinical status of low molecular weight dextran: a critical review. Clin. Pharmacol. Ther. 6:656–665 (1965).
38. J. P. Draye, B. Delaey, A. Van de Voorde, A. Van Den Bulcke, B. Bogdanov, and E. Schacht. In vitro release characteristics of bioactive molecules from dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials 19:99–107 (1998).
39. I. S. Kim, Y. I. Jeong, and S. H. Kim. Self-assembled hydrogel nanoparticles composed of dextran and poly(ethylene glycol) macromer. Int. J. Pharm. 205:109–116 (2000).
40. Y. Zhang and C. C. Chu. Biodegradable dextran-polylactide hydrogel networks: their swelling, morphology and the controlled release of indomethacin. J. Biomed. Mater. Res. 59:318–328 (2002).
41. R. Mehvar. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J. Control. Rel. 69:1–25 (2000).
42. V. Pade and S. Stavchansky. Estimation of the relative contribution of the transcellular and paracellular pathway to the transport of passively absorbed drugs in the Caco-2 cell culture model. Pharm. Res. 14:1210–1215 (1997).
43. K. L. Audus, R. L. Bartel, I. J. Hidalgo, and R. T. Borchardt. The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm. Res. 7:435–451 (1990).
44. I. J. Hidalgo, T. J. Raub, and R. T. Borchardt. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749 (1989).
45. J. N. Cogburn, M. G. Donovan, and C. S. Schasteen. A model of human small intestinal absorptive cells. 1. Transport barrier. Pharm. Res. 8:210–216 (1991).
46. F. Delie and R. Werner. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug Carrier Syst. 14:221–286 (1997).
47. P. Artursson, K. Palm, and K. Luthman. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46:27–43 (2001).
48. K. I. Hosoya, K. J. Kim, and V. H. Lee. Age-dependent expression of P-glycoprotein gp170 in Caco-2 cell monolayers. Pharm. Res. 13:885–890 (1996).
49. J. Hunter and B. H. Hirst. Intestinal secretion of drugs. The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv. Drug Deliv. Rev. 25:129–157 (1997).
50. P. Artursson and R. T. Borchardt. Intestinal drug absorption and metabolism in cell cultures: Caco-2 and beyond. Pharm. Res. 14: 1655–1658 (1997).
51. G. krishna. K. J. Chen, C. C. Lin and A. A. Nomeir. Permeability of lipophilic compounds in drug discovery using in-vitro human absorption model, Caco-2. Int. J. Pharm. 222:77–89 (2001).
52. W. Kamm, A. Jonczyk, T. Jung, G. Luckenbach, P. Raddatz, and T. Kissel. Evaluation of absorption enhancement for a potent cyclopeptidic alpha(nu)beta(3)-antagonist in a human intestinal cell line (Caco-2). Eur. J. Pharm. Sci. 10:205–214 (2000).
53. F. Faassen, J. Kelder, J. Lenders, R. Onderwater, and H. Vromans. Physicochemical properties and transport of steroids across Caco-2 cells. Pharm. Res. 20:177–186 (2003).
54. P. Artursson, and J. Karlsson. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 175:880–885 (1991).
55. S. Yee. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man: fact or myth. Pharm. Res. 14:763–766 (1997).
56. S. Yamashita, Y. Tanaka, Y. Endoh, Y. Taki, T. Sakane, T. Nadai, and H. Sezaki. Analysis of drug permeation across Caco-2 monolayer: implication for predicting in vivo drug absorption. Pharm. Res. 14:486–491 (1997).
57. Y. Tezuka, K. Imai, M. Oshima, and T. Chiba. Determination of substituent distribution in cellulose ethers by 13C- and 1H-NMR studies of their acetylated derivatives: O-(2-hydroxypropyl) cellulose. Carbohydr. Res. 196:1–10 (1990).
58. M. G. Wirick and M. H. Waldman. Some solution properties of fractionated water-soluble hydroxypropylcellulose. J. Appl. Polym. Sci. 14:579–597 (1970).
59. M. F. Francis, M. Cristea, and F. M. Winnik. Polymeric micelles for oral drug delivery: why and how. Pure Appl. Chem. 76: 1321–1335 (2004).
60. D. Blakeslee. Immunofluorescence using dichlorotriazinylaminofluorescein (DTAF). II. Preparation, purity and stability of the compound. J. Immunol. Methods 17:361–364 (1977).
61. C. L. Zhao, M. A. Winnik, G. Riess, and M. D. Croucher. Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers. Langmuir 6:514–516 (1990).
62. T. Mosmann. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55–63 (1983).
63. M. B. Hansen, S. E. Nielsen, and K. Berg. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 119:203–210 (1989).
64. C. A. Bailey, P. Bryla, and A. W. Malick. The use of the intestinal epithelial cell culture model, Caco-2, in pharmaceutical development. Adv. Drug Deliv. Rev. 22:85–103 (1996).
65. M. M. Nerurkar, P. S. Burton, and R. T. Borchardt. The use of surfactants to enhance the permeability of peptides through Caco-2 cells by inhibition of an apically polarized efflux system. Pharm. Res. 13:528–534 (1996).
66. E. V. Batrakova, H. Y. Han, V. Y. Alakhov, D. W. Miller, and A. V. Kabanov. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm. Res. 15:850–855 (1998).
67. E. V. Batrakova, H. Y. Han, D. W. Miller, and A. V. Kabanov. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm. Res. 15: 1525–1532 (1998).
68. P. Augustijns, T. P. Bradshaw, L. S. Gan, R. W. Hendren, and D. R. Thakker. Evidence for a polarized efflux system in Caco-2 cells capable of modulating cyclosporin A transport. Biochem. Biophys. Res. Commun. 197:360–365 (1993).
69. G. Fricker, J. F. Drewe, J. Huwyler, H. Gutmann, and C. Beglinger. Relevance of P-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br. J. Pharmacol. 118: 1841–1847 (1996).
70. Y. Y. Chiu, K. Higaki, B. L. Neudeck, J. L. Barnett, L. S. Welage, and G. L. Amidon. Human jejunal permeability of cyclosporin A: influence of surfactants on P-glycoprotein efflux in Caco-2 cells. Pharm. Res. 20:749–756 (2003).
71. D. W. Miller, E. V. Batrakova, T. O. Waltner, V. Y. Alakhov, and A. V. Kabanov. Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjugate Chem. 8:649–657 (1997).
72. V. J. Wacher, J. A. Silverman, Y. Zhang, and L. Z. Benet. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J. Pharm. Sci. 87:1322– 1330 (1998).
73. E. V. Batrakova, S. Li, D. W. Miller, and A. Kabanov. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm. Res. 16: 1366–1372 (1999).
74. R. Wiwattanapatapee, B. Carreno-Gomez, N. Malik, and R. Duncan. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm. Res. 17:991–998 (2000).
75. M. El-Sayed, M. Ginski, C. Rhodes, and H. Ghandehari. Trans-epithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J. Control. Rel. 81:355–365 (2002).
76. M. El-Sayed, C. A. Rhodes, M. Ginski, and H. Ghandehari. Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int. J. Pharm. 265:151–157 (2003).
77. P. Jani, G. W. Halbert, J. Langridge, and A. T. Florence. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 42:821–826 (1990).
78. J. P. Ebel. A method for quantifying particle absorption from the small intestine of the mouse. Pharm. Res. 7:848–851 (1990).
79. L. Simon, G. Shine, and A. D. Dayan. Translocation of particulates across the gut wall - a quantitative approach. J. Drug Target. 3:217–219 (1995).
80. K. E. Carr, R. A. Hazzard, S. Reid, and G. M. Hodges. The effect of size on uptake of orally administered latex microparticles in the small intestine and transport to mesenteric lymph nodes. Pharm. Res. 13:1205–1209 (1996).
81. A. T. Florence and N. Hussain. Trancytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv. Drug Deliv. Rev. 50:S69–S89 (2001).
82. Y. Miyazaki, S. Yakou, T. Nagai, and K. Takayama. Release profiles of theophylline from microspheres consisting of dextran derivatives and cellulose acetate butyrate: effect of polyion complex formation. Drug Dev. Ind. Pharm. 29:795–804 (2003).
83. G. Ponchel. and J. Irache. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev. 34:191–219 (1998).
84. J. K. Vasir, K. Tambwekar, and S. Garg. Bioadhesive micro-spheres as a controlled drug delivery system. Int. J. Pharm. 255: 13–32 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Francis, M., Cristea, M., Yang, Y. et al. Engineering Polysaccharide-Based Polymeric Micelles to Enhance Permeability of Cyclosporin A Across Caco-2 Cells. Pharm Res 22, 209–219 (2005). https://doi.org/10.1007/s11095-004-1188-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1188-0