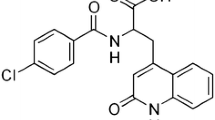
Ebastine (EBS) is a second-generation non-sedating antihistamine used for the prevention and treatment of allergic rhinitis and chronic idiopathic urticaria. It is BCS class II drug exhibiting low aqueous solubility and poor oral bioavailability. The present work was aimed at enhancing the dissolution rate of EBS by formulating it in the form of a liquisolid (LS) system using Tween 20 (non-volatile solvent), Avicel PH 102 (carrier material) and Aerosil 200 (coating material). Various batches of LS powder system were formulated by adopting a mathematical model for calculating required quantities of excipients. The absence of interaction between drug and excipients was checked by Fourier transform IR spectroscopy and differential scanning calorimetry studies. Formulated EBS tablets were evaluated for post compression parameters. X-ray powder diffraction studies and scanning electron microscopy showed the loss of EBS crystallinity in LS formulations. Formulation F9 was considered as optimum, showing a higher drug release of up to 99.06% in comparison to marketed tablet formulations. Stability of the optimized formulation was confirmed by results of the accelerated aging study. Thus, it is concluded that LS formulation is a favorable method of EBS solubility enhancement.









Similar content being viewed by others
References
J. A. Ahmed, Int. J. Pharm. Pharm. Sci.,2, 1 – 7 (2015).
K. T. Savjani, A. K. Gajjar, and J. K. Savjani, Int. Schol. Res. Netw.,1, 1 – 10 (2012).
P. V. Cauwenberge, T. D. Belder, and L. Sys, Expert Opin. Pharmacother.,5, 1807 – 1813 (2004).
M. Dhall and A. K. Madan, J. Incl. Phenom. Macrocycl. Chem.,4, 35 – 45 (2017).
R. R. Kamisetti and V. R. Gupta, Int. J. Pharm. Sci. Nanotech.,10, 3779 – 3787 (2017).
P. R. Rao and G. C. Rao, Int. J. Res. Pharm. Sci.,4, 316 – 320 (2016).
A. Raj and S. Sreerekha, Innorig. Int. J. Sci.,16, 4 – 8 (2015).
M. Lu, H. Xing, J. Jiang, et al., Asian J. Pharm. Sci.,12, 115 – 123 (2017).
M. S. Dahivadkar, H. K. Jain, and K. N. Gujar, Int. Res. J. Pharm., 4, 201 – 204 (2013).
J. J. Savsani, P. P. Goti, and P. B. Patel, Int. J. Chemtech. Res.,5, 47 – 55 (2013).
E. Pavani, S. Noman, and I. A. Syed, Drug Invent. Toda.,5, 302 – 310 (2013).
N. Chella, N. Shastri, and R. R. Tadikonda, Acta. Pharm. Sin. B,2, 502 – 508 (2012).
D. Walunj, Y. Sharma, S. Rawat, and K. Bhise, Int. J. Pharm. Bio. Sci.,3, 591 – 603 (2012).
A. S. Kulkarni, N. H. Aloorkar, M. S. Mane, and J. B. Gaja, Int. J. Pharm. Sci. Nanotech., 3, 795 – 802 (2010).
US Patent, No. 005800834A (1998).
F. J. Sayyad, S. L. Tulsankar, and U. B. Kolap, J. Pharm. Res.,7, 381 – 388 (2013).
Y. Javadzadeh, M. R. Siahi-Shadbad, M. Barzegar-Jalali, and A. Nokhodchi, Il Farm.,60, 361 – 365 (2005).
J. D. Pawar, R. S. Jagtap, R. C. Doijad, et al., J. Drug Deliv. Ther.,7, 6 – 11 (2017).
M. E. Aulton, Powder Flow, in: The Design and Manufacture of Medicines, Churchill Livingstone, Edinburgh (2013), pp. 187 – 199.
L. Lachman, H. A. Lieberman, and J. L. Kanig, in: The Theory and Practice of Industrial Pharmacy, K. E. Avis (Ed.), Varghese Publishing House, Bombay (1991), pp. 183 – 184.
J. D. Pawar, R. S. Jagtap, R. C. Doijad, et al., Asian J. Sci. Technol.,8, 5894 – 5899 (2017).
L. Lachman, H. A. Lieberman, and J. L. Kanig, Tablets, in: The Theory and Practice of Industrial Pharmacy, K. E. Avis (Ed.), Varghese Publishing House, Bombay (1991), pp. 296 – 302.
D. S. Patel, R. M. Pipaliya, and N. Surti, Indian J. Pharm. Sci., 77, 290 – 298 (2015).
B. Haritha, J. Formul. Sci. Bioavailab.,1, 1 – 5 (2017).
Japanese Pharmacopoeia, 16th Edition, Monograph of Ebastine, Pharmaceuticals and Medical Devices Agency (2011), pp. 763 – 764.
International Conference on Harmonization, ICH, Q1A (R2), IFPMA, Geneva (2005).
L. Kumar, M. S. Reddy, R. Verma, et al., Latin Am. J. Pharm.,35(2), 284 – 290 (2016).
N. S. K. Srinivas, R. Verma, G. P. Kulyadi, et al., Int. J. Nanomed., 12, 15 – 28 (2017).
ACKNOWLEDGMENTS
The authors are grateful to Goa College of Pharmacy, Goa, for providing the necessary facilities to carry out this research work.
CONFLICT OF INTEREST
The authors declare that they have no any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harmalkar, D., Godinho, S., Bhide, P.J. et al. New Formulation Technique for Solubility and Dissolution Rate Enhancement of Poorly Soluble Drugs. Pharm Chem J 53, 720–729 (2019). https://doi.org/10.1007/s11094-019-02069-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02069-x