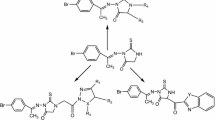
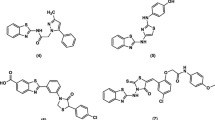
Our previously reported compound, 3-(2-hydroxy-3,4-dimethoxyphenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1), was allowed to react with acetophenone, ethyl cyanoacetate and/or malononitrile to give the corresponding compounds 2a, 2b and 2c, respectively. Treatment of compounds 2b and 2c with thiourea afforded thiopyrimidine derivatives 3a and 3b, respectively. The coupling of 3a and 3b with 2′,3′,4′,6′-tetra-O-acetyl-_-D-glucopyranosyl bromide (4) afforded compounds 5a and 5b, respectively. Reaction of compound 2c with acetophenone yielded pyridone derivative 6, which was fused in pyridine hydrochloride to give demethylated product 7. The coupling of compound 6 with some cyclic and acyclic halosugars afforded various N-glycoside derivatives (8, 9, 11, 13, and 14). New compounds were tested for their antitumor activity on MCF-7 human breast adenocarcinoma cell line and HepG2 liver carcinoma cell line. Almost all tested compounds exhibited antitumor activity, especially 4-(3-(2-hydroxy-3,4-dimethoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)-2-oxo-6-phenyl-1,2-dihydro-pyridine-3-carbonitrile (6) which displayed the most potent inhibitory activity with IC50 = 2.97 and 2.67 g/mL against MCF-7 and HepG2 cell lines, respectively. Compound 6 was tested for its acute toxicity (lethal dose) and found to have very low toxicity based on LD50 values (no label > 600 < 2000 mg/kg) as recommended by the Organization for Economic Co-operation and Development.




Similar content being viewed by others
References
H. Wu, D. Chang, and C. Huang, J. Cancer Mol., 2, 57 – 66 (2006).
J. R. Sierra, V. Cepero, and S. Giordano, Mol. Cancer, 9, 75 – 88 (2010).
L. B. Riley and D. C. Desai, Surg. Clin. N. Am., 89, 1 – 15 (2009).
K. Nakagawa-Goto, J. H. Wu, and K. H. Lee, Synth. Commun., 35, 1735 – 1739 (2005).
L. Zhang, B. H. Zhang, and D. Y. Zhu, Synth. React. Inorg. Metalorg. Nano-Metal Chem., 38, 742 – 745 (2008).
D. F. Nurit, L. Candice, L. R. Amanda, et al., Eur. J. Med. Chem., 46, 4573 – 4583 (2011).
D. K. Goette, Acad. Dermatol., 4, 633 – 649 (1981).
P. Kwan and M. J. Brodie, Epilepsia, 45, 1141 – 1149 (2004).
E. Ashley, R. McGready, S. Proux, and F. Nosten, Travel Med. Infect. Disease, 4, 159 – 173 (2006).
H. Foks, D. Pancehowska-Ksepko, A. Kedzia, et al., Il Farmaco, 60, 513 – 517 (2005).
R. R. Kumar, S. Perumal, P. Senthilkumar, et al., Eur. J. Med. Chem., 44, 3821 – 3839 (2009).
N. A. Abdel-Latif, N. M. Sabry, A. M. Mohamed, and M. M. Abulla, Monatsh. Chem., 138, 715 – 724 (2007).
B. B. Subudhi, P. K. Panda, S. P. Swain, and P. Sarangi, Acta Polon. Pharm. Drug Res., 66, 147 – 153 (2009).
M. T. Cocco, C. Congiu, V. Lilliu, and V. Onnis, Bioorg. Med. Chem., 15, 1859 – 1867 (2007).
N. M. A. El-Ebiary, R. H. Swellem, A. M. Mossa, and G. A. M. Nawwar, Arch. Pharm. Chem. Life Sci., 9, 528 – 534 (2010).
N. M. A. El-Ebiary, E. M. El-Telbani, R. H. Swellem, et al., Lett. Drug Design Discov., 10 , 444 – 452 (2013).
I. C. Badhwar and K. Venkataraman, Org. Synth., 14, 40 – 41(1934).
W. Baker, J. Chem. Soc., 662 – 670 (1941).
W. J. Horton and J. T. Spence, J. Amer. Chem. Soc., 77, 2894 – 2897(1955).
T. Padmaja, G. D. R. Payani, and V. Padmavathi, Eur. J. Med. Chem., 44, 4557 – 4566 (2009).
H. A. Abd El Salam, E. M. A. Yakout, M. A. El-Hashash, and G. A. M. Nawwar, Monatsh. Chem., 144, 1893 – 1901 (2013).
Organization for Economic Co-operation and Development (OCED), Guideline 401: Acute Oral Toxicity, Paris, OCED (1981).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Ebiary, N.M., Swellem, R.H. & Nawwar, G.A.M. Design, Synthesis and Anticancer Activity of Aza Heterocycles Containing Gallate Moiety (Part III). Pharm Chem J 51, 39–48 (2017). https://doi.org/10.1007/s11094-017-1554-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1554-y