Abstract
The oligomerization of amino acids is an essential process in the chemical evolution of proteins, which are precursors to life on Earth. Although some researchers have observed peptide formation on clay mineral surfaces, the mechanism of peptide bond formation on the clay mineral surface has not been clarified. In this study, the thermal behavior of glycine (Gly) adsorbed on montmorillonite was observed during heating experiments conducted at 150 °C for 336 h under dry, wet, and dry–wet conditions to clarify the mechanism. Approximately 13.9 % of the Gly monomers became peptides on montmorillonite under dry conditions, with diketopiperazine (cyclic dimer) being the main product. On the other hand, peptides were not synthesized in the absence of montmorillonite. Results of IR analysis showed that the Gly monomer was mainly adsorbed via hydrogen bonding between the positively charged amino groups and negatively charged surface sites (i.e., Lewis base sites) on the montmorillonite surface, indicating that the Lewis base site acts as a catalyst for peptide formation. In contrast, peptides were not detected on montmorillonite heated under wet conditions, since excess water shifted the equilibrium towards hydrolysis of the peptides. The presence of water is likely to control thermodynamic peptide production, and clay minerals, especially those with electrophilic defect sites, seem to act as a kinetic catalyst for the peptide formation reaction.
Similar content being viewed by others
Introduction
The processes of emergence and evolution of life on the early Earth are still undefined. The formation of organic polymers is an essential process to produce the precursors of life that lead to the production of proteins, DNA and RNA. The oligomerization of amino acids, i.e., peptide formation (n amino acids → peptide + n − 1 H2O), is an important reaction to produce primitive proteins (Miller and Bada 1988). Therefore, it is of interest to determine the physico-chemical conditions that promote the oligomerization reaction to better understand the conditions on primitive Earth when life was born.
High temperature conditions are thermodynamically favorable for peptide formation because the reaction is endothermic (Shock 1992; Lemke et al. 2009). Protein-like polymers (proteinoids) are synthesized through melting of an anhydrous mixture containing acidic and basic amino acids, such as aspartic acid, glutamic acid and lysine, at high temperature (120–200 °C) (Harada and Fox 1960; Fox and Nakashima 1967; Melius and Srisomsap 1997). However, the proteinoids only contain a small amount of peptide bonds, and they are mainly composed of ester-like bonds (Andini et al. 1975; Fitz et al. 2007). Short peptides have been synthesized by heating a solution containing highly concentrated amino acids in a flow reactor, which simulates the rapid heating and cooling conditions of hydrothermal vents (Imai et al. 1999; Cleaves et al. 2009). However, the formation rates were very low (<1 %) and the peptides were hydrolyzed by rapid hydrolysis (Qian et al. 1993; Bada et al. 1995).
A catalyst is necessary to simulate peptide formation on primitive Earth (Lambert 2008). For example, the copper ion is an effective catalyst to elongate peptides in dry–wet cycle experiments (Schwendinger and Rode 1992; Suwannachot and Rode 1998; Rode 1999). Napier and Yin (2006) reported that the peptide formation rate was highest when the molar ratio of amino acids to copper ions was 2:1, indicating that the catalytic intermediate is a coordinative complex between one copper ion and two amino acid molecules (Suwannachot and Rode 1998; Eder and Rode 1994).
Minerals are also efficient catalysts for peptide formation reactions (Lambert 2008). The behavior of amino acids on mineral surfaces has been investigated since the possibility of peptide formation on clay mineral surfaces was proposed by Bernal (1951). Clay minerals are good candidates for the production of the biopolymer because of the strong affinity of amino acids for the high specific surface area (Greenland et al. 1964; Hedges and Hare 1987; Benetoli et al. 2007). Some researchers have observed short peptide formations on clay minerals in dry–wet cycle experiments (Lahav et al. 1978; Bujdak and Rode 1996, 1999a; Ferris et al. 1996; Son et al. 1998; Brack 2013). They found that peptide formation was promoted by the adsorption of amino acids on clay mineral surfaces and particle edges. However, the mechanisms for specific peptide formation from adsorbed amino acids are unclear because the adsorption conditions of amino acids on clay minerals cannot be directly determined and are still a matter of debate.
In this study, the thermal behavior of glycine (Gly) adsorbed on montmorillonite was observed when heated to 150 °C. Montmorillonite is a smectite group mineral that has a 2:1 layer structure with a high surface area and a high exchangeable cation capacity. Gly is the simplest neutral amino acid (H2N–CH2–COOH) and is achiral. Gly is the most abundantly produced prebiotic amino acid (Miller 1953; Kobayashi et al. 1990; Zaia et al. 2008) and has high reactivity (Schwendinger and Rode 1992; Suwannachot and Rode 1998). The roles of water and clay minerals in peptide formation were evaluated based on the experimental results, and possible conditions where peptide formation first occurred on Earth are proposed in relation to the chemical evolution of primitive life.
Experimental
Materials
Glycine (pure grade: 99.0 %), glycylglycine (Gly2, pure grade: 98 %), glycylglycylglycine (Gly3, pure grade: 99 %) and 2.5-piperazine dione (DKP, pure grade: 99 %) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Montmorillonite was purchased from The Clay Science Society of Japan (Okayama, Japan). The composition of the montmorillonite was (Na0.90 K0.37Ca0.13)[(Fe0.30Al2.77 Mg0.93)(Al0.55Si7.17)O20](OH)4⋅nH2O, and small amounts of quartz and cristobalite were present as impurities based on the information of the mineral. The cation exchange capacity of the montmorillonite was 86.4 meq/100 g, and the Brunauer–Emmett–Teller surface area was estimated to be 27.35 m2/g (Miyawaki et al. 2010). Before the experiments, the montmorillonite was reacted with 1 M NaCl solution to saturate in the exchangeable layer with Na+, and washed repeatedly with ultrapure water to remove dissolved salts in the reacted solution. The removal of chloride was tested using AgNO3.
Experimental
To adsorb Gly on montmorillonite, 3.0 g of the prepared Na-montmorillonite sample was stirred into 30 mL of 0.1 or 1 M Gly solution in a Teflon centrifuge tube (50 cm3) and then the tube was shaken at room temperature for 2 h. The solid was separated by centrifugation (12,000 × g for 20 min), washed twice with 30 mL of ultrapure water, and dried in a vacuum. Hereafter, the Gly treated montmorillonite is called Gly-montmorillonite.
Approximately 0.1 mg Gly-montmorillonite was placed in 2 cm3 glass ampoule. Ultrapure water (0.1 mL) was added to some samples, and the ampoule was flushed with Ar and sealed. Hereafter, the conditions with and without the addition of ultrapure water are referred to the wet and dry conditions, respectively. It should be noted that 4–5 wt.% water was retained Gly-montmorillonite after vacuum drying. Thus, some water was present in the system even under the dry conditions. The prepared ampoules were kept in a drying oven at 150 °C for 336 h. For the dry–wet experiments, the montmorillonite was heated in the ampoule without additional water for 168 h in a drying oven at 150 °C. Then, 0.1 mL water was added in the ampoule and it was sealed and heated again for 168 h under the same conditions (total heating time was 336 h). Five samples were prepared for each set of conditions.
After heating, the samples were freeze-dried and the reactant was replaced with 4 mL of a 0.1 M CaCl2 solution in a 4 cm3 glass ampoule. The ampoule was sealed in Ar atmosphere and left at room temperature for 24 h to desorb Gly and its peptides from the montmorillonite. This procedure is frequently used to desorb amino acids and peptides adsorbed on clay minerals (Lambert 2008). Bujdak and Rode (1999b) tested the efficiency of CaCl2 solution to detach adsorbed amino acids and peptides. They demonstrated that the difference between the concentrations of the added and desorbed amino acids was not significant, and IR peaks derived from the remaining amino acids on the clay mineral surface were not detected after the treatment with CaCl2 solution. The reacted CaCl2 solution was filtered through a polytetrafluoroethylene membrane filter (0.2 μm) using a disposable syringe. The desorbed Gly monomer and peptides were quantified using a high-performance liquid chromatograph (HPLC) as described below. The error associated with the desorption procedure was within ± 0.4 %.
Analysis
Speciation and Quantification of Gly and the Peptides
The Gly monomer was quantified by HPLC using postcolumn ortho-phthalaldehyde derivation (e.g., Benson and Hare 1975). Gly was separated through a cation exchange resin (Hitachi, #2619PH, 4.0 × 150 mm i.d.). The eluent was a citrate buffer solution (3-sodium citrate, citric acid, sodium chloride, and ethanol), and the flow rate was 0.4 mL/min at 60 °C. The separated Gly was derivatized by boric acid buffer solution containing 6 mM ortho-phthalaldehyde, and the Gly derivative was detected by a GL-7453 fluorometric detector (GL Science Inc., Tokyo, Japan) (excitation wavelength: 360 nm; emission wavelength: 440 nm). Gly standard (50 μM) was chromatographed to determine the retention time and to draw the calibration curve.
The glycine peptides were separated using a GL Sciences InertSustain C18 column (length: 4.6 × 250 mm), and analyzed by a UV detector (GL-7450, GL Science) at 195 nm absorbance. The eluent was a mixture of 10 mM KH2PO4 and 7.2 mM C6H13SO3Na, adjusted to pH 2.5 using H3PO4. The flow rate of the mobile phase was 1.0 mL/min at 40 °C. DKP, Gly2, and Gly3 formed were identified by co-injection with synthetic standards. Figure 1a and b show examples of the chromatograms of standard and sample solutions, respectively.
X-Ray Diffraction and Fourier Transform Infrared Spectroscopy
X-ray diffraction (XRD) and Fourier transform infrared (FT-IR) spectroscopy were used to confirm that Gly adsorbed on the exchangeable layer of montmorillonite. XRD (Rigaku Geigerflex RAD-IA, Tokyo, Japan) was carried out to measure the basal layer (001) spacing of the Gly-montmorillonite, using Ni-filtered monochromatic CuKα radiation with 2θ angle between 2° and 14°.
The infrared (IR) spectra were obtained using a FT-IR 8100 spectrometer (Shimadzu Corp, Kyoto, Japan). For the IR spectroscopy, a mixture of 2 mg sample and 100 mg KBr was pressed to make an optically transparent pellet. The pellets were scanned 95 times at a spectral resolution of 8 cm−1 from 400 to 4,000 cm−1.
Results
Changes in Gly Monomer Concentration on Montmorillonite
The amount of Gly adsorbed on the montmorillonite was 234.7 μmol/g when the montmorillonite was treated with 0.1 M Gly solution. The concentration of Gly monomers remaining on the montmorillonite gradually decreased with increasing heating time (Fig. 2). As shown in Table 1, after heating for 336 h, 45.6 % of the Gly remained adsorbed on montmorillonite under dry conditions, while in the wet conditions, 73.3 % of the Gly was still adsorbed on the montmorillonite.
As a reference, Gly was heated without montmorillonite, and 99.1 % and 93.8 % of Gly remained under dry and wet conditions, respectively.
Peptide Formation
Figure 3 shows the changes in the yield of Gly peptides (ratio of produced peptide/initial Gly in %) as a function of heating time, and Table 1 shows the yield after heating for 336 h. Three types of peptides (DKP, Gly2, and Gly3) were identified to have formed with montmorillonite under dry conditions (Fig. 3a), while the peptides were not detected in the wet system.
The amount of Gly peptides on montmorillonite gradually increased with heating time, and a total of 13.9 % of the Gly formed peptides in the dry system after 336 h. DKP was consistently the main product and the yields of Gly2 and Gly3 were low.
When Gly was heated without montmorillonite, 0.7 % of the Gly formed peptides in the wet system after 336 h (Fig. 3b), while no peptides were found under the dry conditions. Although a small amount of DKP and Gly2 were synthesized, Gly3 was not detected when only Gly was heated with water.
Dry–Wet Experiment
49.7 % of the Gly monomer remained when the dry–wet conditions experiment with montmorillonite was finished, and 0.7 % of it transformed into peptides. Most of the DKP was decomposed when the system was heated again with additional water, and Gly2 became the most abundant peptide under these conditions.
XRD and FT-IR Analysis
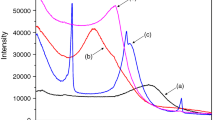
Figure 4 shows the X-ray diffractograms of Na− and Gly-montmorillonite. The basal spacing of Na-montmorillonite (001) was 12.8 Å (Fig. 4a). After shaking in solutions of 0.1 M and 1 M Gly and freeze-drying, the basal spacing of Gly-montmorillonite increased to 13.2 (Fig. 4b) and 16.1 Å (Fig. 4c), respectively (Table 2).
Figure 5a shows the FT-IR spectra of the studied montmorillonites. The band at 1,035 cm−1 corresponds to the Si–O–Si stretching vibration. The band at 3,618 cm−1 is from OH stretching, and the bands at 3,421 and 1,627 cm−1 are from the stretching and bending vibrations of adsorbed water (Fig. 5a(b)) (Naidja and Huang 1994; Cuadros et al. 2009). Gly-montmorillonite has bands between 1,000 and 2,000 cm−1 from the asymmetric deformation of NH3 + (δas) (1,593 cm−1), the symmetric deformation of NH3 + (δs) (1,506 cm−1), the symmetric stretching of COO− (νs) (1,404 cm−1) and the rocking of CH2 (ρr) (1,339 cm−1) (Fig. 5a(a)) (Sato 1999; Kollar et al. 2003). Figure 5b shows an enlargement of the spectra between 1,200 and 2,000 cm−1. The Gly-montmorillonite has bands at 1,634, 1,523, 1,412 and 1,331 cm−1, respectively (Fig. 5b(c)). The band of NH3 + (δas) at 1,634 cm−1 overlaps with the band of OH at 1,627 cm−1. The peak area of the band at 1,627 cm−1 is 306.4, but it considerably increases to 2,075.8 after Gly adsorption. After heating for 288 h under the dry conditions, bands are observed at 1,637, 1,522, 1,419, and 1,334 cm−1 (Fig. 5b(d)). An additional band appears at 1,671 cm−1, which corresponds to the carbonyl band (–C(=O)–) of DKP (Lin et al. 2002). When reacted under wet conditions, the product has bands of NH3 + (δas) at 1,637 cm−1, NH3 + (δs) at 1,522 cm−1, COO− (νs) at 1,419 cm−1 and CH2 (ρr) at 1,338 cm−1 (Fig. 5b(e)).
a FT-IR spectra between 400 and 4,000 cm−1. (a) Gly monomer, (b) Na-montmorillonite, (c) Gly-montmorillonite, (d) Gly-montmorillonite after heating at 150 °C under dry conditions and (e) Gly-montmorillonite after heating at 150 °C under wet conditions for 228 h. In (a), the band at 1,593 cm−1 represent asymmetric deformation of NH3 + (δas), the band at 1,506 cm−1 is symmetric deformation of NH3 + (δs), the band at 1,404 cm−1 is symmetric stretching of COO− (νs) and the band at 1,339 cm−1 is rocking of CH2 (ρr) of pure Gly. b The spectra (b), (c), (d) and (e) extended between 1,200 and 2,000 cm−1
Discussion
Peptide Bond Formation of Gly During Adsorption on Montmorillonite
Amino acids commonly adsorb to the interlayer of smectite group minerals (Greenland et al. 1964). The basal spacing (001) expands when amino acids occupy the interlayer space of montmorillonite (Fig. 4 and Table 2) (Barshad 1952; Naidja and Huang 1994). The interlayer spacing of the Na-montmorillonite interlayer increased from 12.8 Å to 13.2 and 16.1 Å after shaking in solutions of 0.1 M and 1 M Gly respectively, confirming that Gly moved from solution into the interlayer. Gly molecules weakly adsorbed on the surface and edges of montmorillonite, and then might have desorbed when the montmorillonite was washed twice with ultra-pure water because the activation energy for desorption is small (Lambert 2008). Amino acids more strongly adsorb in the interlayer than on the surface (Friebele et al. 1980). Under the conditions of this study, most of the Gly is expected to remain in the interlayer as interlayer cations.
Amino acids occupy the interlayer of smectite minerals via cation exchange (Greenland et al. 1964; Naidja and Huang 1994). Friebele et al. (1980) observed changes in the amount of Gly and alanine (Ala) adsorbed on Na-montmorillonite at pH 3–10, and found that the amount of adsorbed amino acids markedly increased at pH 3, i.e., when these amino acids exist as cations. Thus, cation exchange would be the main mechanism for amino acid adsorption to the interlayer. However, the majority of Gly in our studied solution existed as zwitterions rather than cations, since the pH of the Gly solution was 6.4 (2.3 < pK < 9.6). Parbhakar et al. (2007) suggested that proton transfer was an important mechanism for amino acid zwitterions to occupy the interlayers. The reaction is described as
The amino acid zwitterions would change to cation in the interlayer via proton transfer. The FT-IR spectra indicated that interlayer Gly was in a cationic state. The ratio of the peak areas of δas NH3 + before and after adsorption on montmorillonite did not greatly decrease (0.85), while that for νs COO− considerably decreased (0.49). In addition, proton transfer in the interlayer space must have increased the pH of the solution after shaking owing to dissociation of H2O and dissolution of OH−. In fact, the pH value of the Gly solution increased from 6.3 to 7.3 (±0.1) after shaking with Na-montmorillonite. Thus, proton transfer could have been important to substitute interlayer cations with Gly in this study.
Interlayer amino acids are likely to adsorb by hydrogen bonding interactions based on the IR bands of the amino acids (Naidja et al. 1995; Kollar et al. 2003; Stievano et al. 2007; Kitadai et al. 2009). Sato (1999) suggested that the peaks of the amino groups shifted to higher wave numbers when Gly was adsorbed in the interlayer of clay minerals via the protonated amino group (NH3 +). In this study, the IR bands for COO− (νs) and CH2 (ρr) slightly shifted, while those for NH3 + (δas) and NH3 + (δs) significantly shifted from 1,593 to 1,634 cm−1 and 1,506 to 1,523 cm−1, respectively (Fig. 5b). The band of the amino group at 1,593 cm−1 shifted to a higher wave number, suggesting that the protonated amino group (NH3 +) played a role in the adsorption. The protonated amino group is an excellent hydrogen bond donor (Marsh and Donohue 1967) and forms a hydrogen bond with the structural oxygen atoms of mineral surfaces (Raussel-Colom and Salvador 1971; Naidja and Huang 1994). Thus, the possible adsorption mechanism of Gly in the interlayer of montmorillonite would be mainly hydrogen bond between the protonated amino group of Gly (NH3 +) and Si–O− and Al–O− groups on the montmorillonite surface.
The thermal behavior of amino acids adsorbed on mineral surfaces via hydrogen bond is not cleared. Kanamura and Vand (1970) suggested that the hydrogen bond may stabilize the amino acids on the mineral surface. Cuadros et al. (2009) observed hydrogen bond formation between lysine and the montmorillonite surface under hydrothermal conditions (80 °C), and estimated that the hydrogen bond became stronger and stabilized with increasing temperature. In this study, however, the reactivity of the Gly monomer adsorbed on the montmorillonite increased with increasing temperature, and the amount of Gly monomer more rapidly decreased in the system with montmorillonite than in the system without montmorillonite under both dry and wet conditions. Therefore, the hydrogen bond activated rather than stabilized Gly.
When Gly was heated without montmorillonite, 99 % of the Gly remained and peptides were not detected after 336 h (Table 1), although peptide formation is thermodynamically favorable under high temperature and dry conditions. Crystalline amino acids have strong hydrogen bonds between the protonated amino groups and deprotonated carboxyl groups (NH3 +⋅⋅⋅COO−), which are very stable under high temperature and dry conditions (Rosado et al. 1997; Reva et al. 1994). Sato (1999) suggested that the reactivity of amino acids adsorbed on mineral surface was higher than that of the crystalline amino acids because the strong intermolecular hydrogen bonds of the crystalline amino acid were broken and weaker hydrogen bonds between the amino acids and mineral surface were formed. Thus, the activation process of adsorption would be a determining factor for the success of the first step of peptide formation.
Bujdak and Rode (1996, 1999a) observed oligomerization of Gly on some clay minerals during a dry–wet cycle experiment (80 °C, open system). They estimated that Gly was activated and oligomerized on the clay mineral surface through the formation of an ester bond (Si–O–C(=O)–R) between the carboxyl group of the amino acid (COO−) and the silanol group (Si–OH) on the clay mineral surface and edge. Peptide formation on mineral surfaces is often explained by the nucleophilic reaction between an amino group and a carboxyl group of amino acids. The electron density of the carbon atom of the carboxyl group of the amino acid decreases through adsorption because of ester bond formation and nucleophilic attack at the carbon atom by an amino group of another amino acid (Bujdak and Rode 1996, 1999a; Basiuk and Gromovoy 1993). However, the ester bond cannot be easily formed under 100 °C, because this temperature is thermodynamically unfavorable for the formation reaction (Δ°G = 22.5 to −29.7 kJ mol−1) (Rimola et al. 2006). In fact, the formation of an ester bond is observed only when amino acids are heated with silicate minerals in the dry condition system at very high temperature (>600 °C) (e.g., Meng et al. 2004). An IR peak at 1,760 cm−1 provides evidence of the formation of an ester bond (Basiuk et al. 1990). However, this peak has not been detected in other experiments of the simulation of peptide formation on mineral surfaces (Lomenech et al. 2005), as well as in this study. Thus, peptide formation in this study is likely to be independent of the ester bond formation on montmorillonite.
Recently, some molecular modeling studies using ab initio methods, which are used to calculate and simulate molecular structures based on the first principles of quantum mechanisms, revealed that the acid–base reaction, especially at Lewis and Brønsted acid/base sites on the mineral surface, is an important catalyst for the peptide formation reaction (Yu et al. 2001; Aquino et al. 2004; Rimola et al. 2005, 2007). Clay mineral surfaces have many electrophilic defects. For example, the aluminum atom (Al3+) acts as a Lewis acid or base site when the hydroxyl group of the aluminol group (Al–OH) changes to –O− or –OH2 + by dehydration or change of the pH, respectively (Ward and Brady 1998). Aquino et al. (2004) suggested that electrophilic defect sites, such as the aluminum atom that loses its hydroxyl group (i.e., becomes a Lewis acid site) on the clay mineral surface, were more suitable for the peptide formation reaction than sites covered with a hydroxyl group, such as Si–OH or Al–OH. Rimola et al. (2007) simulated dimerization of Gly on Lewis and Brønsted acid sites on feldspars using ab initio methods, and revealed that the Lewis acid sites (Al3+) act as a catalyst when Gly is adsorbed via the oxygen atom of the carbonyl group. These studies simulated amino acid adsorption via the carboxyl group on a positively charged mineral surface, i.e., Lewis and Brønsted acid sites.
However, our results, and many studies using FT-IR, revealed that hydrogen bond formation between the protonated amino group and the negatively charged mineral surface is the main adsorption mechanism of amino acids. In other words, most of the amino acids were not adsorbed on the acid site but on the base site. Aquino et al. (2004) estimated that the Lewis base sites are also effective catalysts that enhance the peptide formation, because the adsorption of amino acids by hydrogen bond on the Lewis base sites was observed during molecular modeling simulations. Recently, using attenuated total reflection FT-IR spectroscopy, Ramos and Huertas (2013) found that Gly adsorbed by the carboxyl group on montmorillonite edge sites only when the concentration of the Gly solution was very low (<0.001 M) and that hydrogen bond formation was the main adsorption mechanism of amino acids on montmorillonite. In this study, 13.9 % of Gly adsorbed on montmorillonite changed into peptides after heating for 336 h under dry condition, estimating that peptide formation reaction would be enhanced via the hydrogen bond formation on the base site. Thus, we suggest that the base site on montmorillonite is a more important adsorption site for amino acids and catalyst for peptide formation than the acid site.
Dehydration Promotion and DKP Condensation on Montmorillonite Under Dry Conditions
The reactivity of the interlayer Gly of montmorillonite was considerable enhanced by dry conditions, and 40.5 % of Gly disappeared after heated for 336 h. The lost Gly could have decomposed. The decomposition pathways of Gly are divided into deamination, decarboxylation, dehydration and hydrolysis. Of these, deamination and dehydration are the main reaction pathways that occur under high temperature and dry conditions (Li et al. 2007; Otake et al. 2011). The formation of peptides under high temperature and dry conditions in this study suggests that dehydration may be the main decomposition pathway on the montmorillonite surface.
Kitadai et al. (2011) investigated the effects of anhydrous salts, where hydration is thermodynamically favorable, on peptide formation under dry conditions at 140 °C. They reported that 6.92 % of Gly formed peptides when heated for 20 days with MgSO4, but only 0.035 % formed peptides in the absence of MgSO4. Montmorillonite can also promote dehydration, because polar H2O molecules are easily adsorbed on the negatively charged surface (Hensen and Smit 2002). Paecht-Horowitz and Lahav (1977) reported that the amount of newly formed Ala peptides was lower when Ala was reacted with Al-montmorillonite than with Na-montmorillonite because Al-montmorillonite does not have the ability to adsorb water molecules. Under high temperature and dry conditions, clay minerals most likely absorb water, similar to metal and anhydrous inorganic salts, and take water from amino acids, promoting peptide formation. In particular, the minerals from the smectite group, including montmorillonite, enhance the dehydration of amino acids because of their high absorbency.
The reaction pathway of the oligomerization of Gly is shown in Fig. 6. Most of the peptides formed on montmorillonite during the experiment under dry conditions were in the form of DKP (86 % of the total peptides formed after heating for 336 h). DKP is easily formed via cyclization and dehydration of Gly2. Figure 7a shows how the ratio of products (Gly2/DKP) on montmorillonite changed with heating time. The ratio gradually decreased with increasing heating time, indicating that DKP formation from Gly2 (i.e., Gly2 → DKP + H2O) was promoted with increasing heating time. This trend indicates that cyclization of Gly2 (i.e. DKP formation) followed the formation of linear peptides on montmorillonite. DKP formation from amino acid monomers is thermodynamically and kinetically favorable at high temperatures and in dry conditions because the reaction is dehydration (Bujdak and Rode 1996). Thus, DKP was condensed on montmorillonite because of the dehydration promotion under continuous dry condition.
Some studies have often concluded that DKP formation is a dead-end for peptide elongation (Steinberg and Bada 1981; Basiuk et al. 1990). These experiments were performed under continuous thermal dry conditions. On the other hand, Nagayama et al. (1990) heated Gly monomer with DKP under hydrothermal conditions, and observed that amount of Gly3 was increased with heating time. They explained that DKP formation was an important intermediate step to provide the internal free energy necessary to form additional peptide bonds, and that the peptide was elongated via the opening of the DKP ring and subsequent bonding to another amino group. The DKP molecule has two peptide bonds, and can oligomerize more easily than Gly2 (Lambert 2008). In this study, production of the linear tripeptide (Gly3) on montmorillonite was limited with increasing heating time under dry conditions. Since the DKP ring has a thermally stable molecular structure, it would not easily open under high temperature and dry conditions. Thus, DKP must be a dead-end for peptide elongation under continuous dry condition. However, peptides could be elongated to more than trimer via hydrolysis of DKP (i.e., DKP + H2O → Gly2) if sufficient water exists.
Effect of Water on Hydrolysis of Peptides on Montmorillonite
The reactivity of Gly on montmorillonite interlayers under wet conditions was lower than under dry conditions. The rate of dehydration is controlled by the water content of the system. The hydrolysis of Gly and peptides is the main reaction that occurs under wet condition, i.e., in presence of excess water.
Although Gly was hardly decreased without montmorillonite, 26.7 % of Gly adsorbed on montmorillonite disappeared after heated for 336 h. The lost Gly could have hydrolyzed.
A small amount of short peptides (<1 %) can form under simulated hydrothermal conditions without a catalyst (Imai et al. 1999; Cleaves et al. 2009; Lemke et al. 2009). Our results are in agreement with those reports: 0.7 % of Gly changed into peptides under wet conditions in the absence of montmorillonite. Most of the peptide formed on montmorillonite under dry conditions was DKP, while it was 59 % under the wet condition. Figure 7b shows how the ratio of products (Gly2/DKP) changed with heating time under the wet condition. The product ratio of Gly2/DKP increased with heating time, suggesting that cyclization of Gly2 (i.e., Gly2 → DKP + H2O) was controlled by excess water.
On the other hand, no peptides formed in the wet system with montmorillonite. As mentioned above, peptide formation is likely to be promoted through adsorption on the Lewis base sites of montmorillonite. The Lewis base site is formed through dehydration of aluminol and silanol groups. However, extra water molecules are usually coordinated to the aluminum atoms when excess water is present (Rimola et al. 2007). Thus, there should be less electrophilic defect sites under wet conditions than under dry conditions. Therefore, the peptide formation reaction may not be enhanced on montmorillonite under wet conditions because of hard dehydration.
In addition, hydrolysis of peptides would be promoted on mineral surface as well as its formation under wet conditions (Marshall-Bowman et al. 2010; Schreiner et al. 2011). The lower concentration of peptides on montmorillonite would be caused because of the rapid peptide hydrolysis. Although sufficient water is need to open the DKP rings, excessive water promoted hydrolysis of DKP beyond opening of the rings. Thus, continuous wet condition is likely to inadequate to condense and elongate peptides.
It has been observed that peptide elongation is promoted under the conditions of dry–wet cycling in the presence of clay minerals (Lahav et al. 1978; Bujdak and Rode 1999a). Bujdak and Rode (1999a) heated Gly2 with some clay minerals under dry–wet cycling conditions at 80 °C, and observed linear peptide (Gly4) formation. They explained that linear peptide elongation from DKP was dependent on the water activity, and dry–wet cycling provided the conditions of high water activity. In this study, dry–wet conditions were investigated to observe the behavior of DKP formed on montmorillonite heated at 150 °C. In this study, water added was not evaporated, although previous studies used open system. After addition of water to the system, the amount of Gly remaining on montmorillonite considerably decreased (Table 1). Most of DKP was decomposed after the addition of water. However, long peptides, such as Gly3 and Gly4, were not formed, indicating evaporation process is essential for peptide elongation.
Based on our results, continuous dry and wet conditions are inadequate to form long peptides, although clay minerals are important catalyst to activate amino acids. The conditions required for chemical evolution of amino acids are likely to be those where hydration–dehydration effectively occurred, such as lagoons and tidal flats on the primitive Earth.
Conclusions
-
(1)
Gly was easily adsorbed on the interlayer of montmorillonite and its reactivity increased after adsorption. The yield of Gly peptides formed on montmorillonite was higher when the reaction occurred under dry rather than wet conditions, and DKP was the main product. Based on the results of IR analysis, the Gly monomer mainly adsorbed via hydrogen bonding between the positively charged amino group and the negatively charged site (i.e., Lewis base site) on the montmorillonite surface, suggesting that the Lewis base site enhances peptide formation.
-
(2)
Clay minerals are important catalysts to activate amino acids. However, a considerable amount of DKP, which is a dead-end for peptide elongation, were formed, and peptides were not elongated under dry conditions. In addition, no peptides formed in the wet system with montmorillonite because of hydrolysis promotion. Based on our results, we concluded that continuous dry and wet conditions are inadequate to form long peptides, and a hydration–dehydration system is likely to be more suitable for chemical evolution of amino acids.
References
Andini S, Benedetti E, Ferrara L, Paolillo L, Temussi PA (1975) NMR-studies of prebiotic polypeptides. Orig Life Evol Biosph 6:147–153
Aquino AJA, Tunega D, Gerzabek MH, Lischka H (2004) Modeling catalytic effects of clay mineral surfaces on peptide bond formation. J Phys Chem B 108:10120–10130
Bada JL, Miller SL, Zhao M (1995) The stability of amino acids at submarine hydrothermal vent temperature. Orig Life Evol Biosph 25:111–118
Barshad I (1952) Factors affecting the interlayer expansion of vermiculite and montmorillonite with organic substances. Soil Sci Soc Am Proc 16:176–182
Basiuk VA, Gromovoy TY (1993) Reactions of vaporous proteinogenic α-amino acids a silica and alumina surfaces. React Kinet Catal Lett 50:297–303
Basiuk VA, Gromovoy TY, Golovaty VG, Glukhoy AM (1990) Mechanism of amino acid polycondensation on silica and alumina surfaces. Orig Life Evol Biosph 20:483–498
Benetoli LOB, Souza CMD, Silva KL, Souza IG, Santana H, Paesano A, Costa ACS, Zaia CTBV, Zaia DAM (2007) Amino acid interaction with and adsorption on clays: FT-IR and Mossbauer spectroscopy and X-ray diffractometry investigations. Orig Life Evol Biosph 37:479–493
Benson JR, Hare PE (1975) Ortho-phthalaldehyde–fluorogenic detection of primary amines in picomole range—comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A 72:612–622
Bernal JD (1951) The physical basis of life. Routledge and Kegan Paul, London
Brack A (2013) Clay minerals and the origin of life. In: Bergaya F, Lagaly G (eds) Handbook of clay science, 2nd edn. Elsevier, Amsterdam, pp 507–521
Bujdak J, Rode BM (1996) The effect of smectite composition on the catalysis of peptide bond formation. J Mol Evol 43:326–333
Bujdak J, Rode BM (1999a) The effect of clay structure on peptide bond formation catalysis. J Mol Catal A 144:129–136
Bujdak J, Rode BM (1999b) Silica, alumina and clay catalyzed peptide bond formation: enhanced efficiency of alumina catalyst. Orig Life Evol Biosph 29:451–461
Cleaves HJ, Aubrey AD, Bada JL (2009) An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig Life Evol Biosph 39:109–126
Cuadros J, Aldega L, Vetterlein J, Drickmer K, Dubbin W (2009) Reactions of lysine with montmorillonite at 80 °C: implication for optical activity, H+ transfer and lysine-montmorillonite binding. J Colloid Interface Sci 333:78–84
Eder AH, Rode BM (1994) Influence of alkali- and alkaline-earth-metal cations on the ‘salt-induced peptide formation’ reaction. J Chem Soc Dalton Trans 8:1125–1130
Ferris JP, Hill AR Jr, Liu R, Orgel LE (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61
Fitz D, Reiner H, Rode BM (2007) Chemical evolution toward the origin of life. Pure Appl Chem 79:2101–2117
Fox SW, Nakashima T (1967) Fractionation and characterization of an amidated thermal 1:1:1-proteinoid. Biochem Biophys Acta 140:155–167
Friebele E, Shimoyama A, Ponnamperuma C (1980) Adsorption of protein and non-protein amino acids on a clay mineral: a possible role of selection in chemical evolution. J Mol Evol 16:269–278
Greenland DJ, Laby RH, Quirk JP (1964) Adsorption of amino-acids and peptides by montmorillonite and illite. Trans Faraday Soc 61:2013–2023
Harada K, Fox SW (1960) The thermal copolymerization of aspartic acid and glutamic acid. Archiv Biochem Biosph 86:274–280
Hedges JI, Hare PE (1987) Amino acid adsorption by clay minerals in distilled water. Geochim Cosmochim Acta 51:255–259
Hensen EJM, Smit B (2002) Why clays swell. J Phys Chem B 106:12664–12667
Imai E, Honda H, Hatori K, Brack A, Matsuno K (1999) Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 283:831–833
Kanamura F, Vand V (1970) The crystal structure of a clay-organic complex of 6-amino hexanoic acid and vermiculite. Am Mineral 55:1550–1561
Kitadai N, Yokoyama T, Nakashima S (2009) In situ ATR-IR investigation of L-lysine adsorption on montmorillonite. J Colloid Interface Sci 338:395–401
Kitadai N, Yokoyama T, Nakashima S (2011) Hydration-dehydration interactions between glycine and anhydrous salts: implications for a chemical evolution of life. Geochim Cosmochim Acta 75:6285–6299
Kobayashi K, Tsuchiya M, Ohshima T, Yanagawa H (1990) Abiotic synthesis of amino-acids and imidazole proton irradiation of simulated primitive earth atmosphere. Orig Life Evol Biosph 20:99–109
Kollar T, Palinko I, Konya Z, Kiricsi I (2003) Intercalating amino acid guests into montmorillonite host. J Mol Struct 651:335–340
Lahav N, White D, Chang S (1978) Peptide formation in the prebiotic era: thermal condensation of glycine in fluctuating clay environments. Science 201:67–69
Lambert JF (2008) Adsorption and polymerization of amino acids on mineral surfaces: a review. Orig Life Evol Biosph 38:211–242
Lemke K, Rosenbauer RJ, Bird DK (2009) Peptide synthesis in early earth hydrothermal systems. Astrobiology 9:141–145
Li J, Wang Z, Yang X, Hu L, Liu Y, Wang C (2007) Evaluate the pyrolysis pathway of glycine and glycilglycine by TG-FTIR. J Anal Appl Pyrolysis 80:247–253
Lin SY, Wang SL, Chen TF, Hu TC (2002) Intramolecular cyclization of diketopiperazine formation in solid-state enalapril maleate studied by thermal FT-IR microscopic system. Eur J Pharm Biopharm 54:249–254
Lomenech C, Bery G, Costa D, Stievano L, Lambert JF (2005) Theoretical and experimental study of the adsorption of neutral glycine on silica from the gas phase. ChemPhysChem 6:1061–1070
Marsh RE, Donohue J (1967) Crystal structure studies of amino acids and peptides. Adv Protein Chem 22:235–256
Marshall-Bowman K, Ohara S, Sverjensky DA, Hazen RM, Cleaves HJ (2010) Catalytic peptide hydrolysis by mineral surface: implications for prebiotic chemistry. Geochim Cosmochim Acta 74:5852–5861
Melius P, Srisomsap C (1997) Sequences in hydrolysates of thermal poly (glutamic acid, phenylalanine, alanine, metionine). Polymer 38:4989–4992
Meng M, Stievano L, Lambert JF (2004) Adsorption and thermal condensation mechanisms of amino acids on oxide supports. 1. Glycine on silica. Langmuir 20:914–923
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117:528–529
Miller SL, Bada JL (1988) Submarine hot springs and the origin of life. Nature 334:609–611
Miyawaki R, Sano T, Ohashi F, Suzuki M, Kogure T, Okumura T, Kameda J, Umezome T, Sato T, Chino D, Hiroyama K, Yamada H, Tamura K, Morimoto K, Uehara S, Hatta T (2010) Some reference date for the JCSS clay specimens. J Clay Sci Soc Jpn 48:158–198
Nagayama M, Takaoka O, Inomata K, Yamagata Y (1990) Diketopiperazine-mediated peptide formation in aqueous solution. Orig Life Evol Biosph 20:249–257
Naidja A, Huang PM (1994) Aspartic acid interaction with Ca-montmorillonite: adsorption, desorption and thermal stability. Appl Clay Sci 9:265–281
Naidja A, Violante A, Huang PM (1995) Adsorption of tyrosinase onto montmorillonite as influenced by hydroxyaluminum coatings. Clays Clay Minerals 43:647–655
Napier J, Yin J (2006) Formation of peptides in the dry state. Peptide 27:607–610
Otake T, Taniguchi T, Furukaw Y, Kawamura F, Nakazawa H, Kakegawa T (2011) Stability of amino acids and their oligomerization under high-pressure conditions: implications for prebiotic chemistry. Astrobiology 11:799–813
Paecht-Horowitz M, Lahav N (1977) Polymerization of alanine in the presence of a non-swelling montmorillonite. J Mol Evol 10:73–76
Parbhakar A, Cuadro J, Sephton MA, Dubbin W, Coles BJ, Weiss D (2007) Adsorption of L-lycine on montmorillonite. Colloids Surf A 307:142–149
Qian Y, Engel MH, Macko SA, Carpenter S, Deming JW (1993) Kinetics of peptide hydrolysis and amino acid decomposition at high temperature. Geochim Cosmochim Acta 57:3281–3293
Ramos ME, Huertas FJ (2013) Adsorption of glycine on montmorillonite in aqueous solutions. Appl Clay Sci 80–81:10–17
Raussel-Colom JA, Salvador PS (1971) Complexes vermiculite-aminoacides. Clay Miner 9:139–149
Reva ID, Stepanian SG, Plokhotnichenko AM, Rodchenko ED, Sheina GG, Blagoi YP (1994) Infrared matrix isolation studies of amino acids. Molecular structure of proline. J Mol Struct 318:1–13
Rimola A, Tosoni S, Sodupe M, Ugliengo P (2005) Peptide bond formation activated by the interplay of Lewis and Brønsted catalysts. Chem Phys Lett 408:295–301
Rimola A, Tosoni S, Sodupe M, Ugliengo P (2006) Dose silica surface catalyse peptide bond formation? New insights from first-principles calculations. ChemPhysChem 7:157–163
Rimola A, Sodupe M, Ugliengo P (2007) Aluminosilicate surfaces as promoters for peptide bond formation: an assessment of Bernal’s hypothesis by ab initio methods. J Am Chem Soc 129:8333–8344
Rode BM (1999) Peptide and the origin of life. Peptides 20:773–786
Rosado MTS, Duarte MLRS, Fausto R (1997) Vibrational spectra (FT-IR, Raman and MI-IR) of α and β-alanine. J Mol Struct 410:343–348
Sato M (1999) Preparation of kaolinite-amino acid intercalates derived from hydrated kaolinite. Clays Clay Minerals 47:793–802
Schreiner E, Nair NN, Wittekindt C, Marx D (2011) Peptide synthesis in aqueous environments: the role of extreme conditions and pyrite mineral surfaces on formation and hydrolysis of peptides. J Am Chem Soc 133:8216–8226
Schwendinger MG, Rode BM (1992) Investigation on the mechanism of the salt-induced peptide formation. Orig Life Evol Biosph 22:349–359
Shock EL (1992) Stability of peptide in high-temperature aqueous solutions. Geochim Cosmochim Acta 56:3481–3491
Son HL, Suwannachot Y, Bujdak J, Rode BM (1998) Salt-induced peptide formation from amino acids in the presence of clays and related catalysts. Inorg Chim Acta 272:89–94
Steinberg S, Bada JL (1981) Diketopiperazine formation during investigations of amino acids racemization in dipeptides. Science 213:544–545
Stievano L, Piao LY, Lopes I, Meng M, Costa D, Lambert JF (2007) Glycine and lysine adsorption and reactivity on the surface of amorphous silica. Eur J Mineral 19:321–331
Suwannachot Y, Rode BM (1998) Catalysis of dialanine formation by glycine in the salt-induced peptide formation reaction. Orig Life Evol Biosph 28:79–90
Ward DB, Brady PV (1998) Effect of Al and organic acids on the surface chemistry of kaolinite. Clays Clay Minerals 46:453–465
Yu CH, Newton SQ, Miller DM, Teppen BJ, Schafer L (2001) Ab initio study of the nonequivalence of adsorption of D- and L-peptides on clay mineral surfaces. Struct Chem 12:393–398
Zaia DAM, Zaia CTBV, Santana HD (2008) Which amino acids should be used in brebiotic chemistry studies? Orig Life Evol Biosph 38:469–488
Acknowledgments
We thank K. Okazaki for HPLC analysis. We would also like to thank E. Even for useful comments. This study was financially supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan through a special coordination fund (Project TAIGA: Trans-crustal Advection and In situ reaction of Global subseafloor Aquifer).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchida, S., Masuda, H. & Shinoda, K. Peptide Formation Mechanism on Montmorillonite Under Thermal Conditions. Orig Life Evol Biosph 44, 13–28 (2014). https://doi.org/10.1007/s11084-014-9359-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-014-9359-4