Abstract
The idea is advanced that under the extreme earth conditions for ~3.9 billions years ago, protein-based β-sheet molecular structures were the first self-propagating and information-processing biomolecules that evolved. The amyloid structure of these aggregates provided an effective protection against the harsh conditions known to decompose both polyribonucleotides and natively folded polypeptides. In the prebiotic amyloid world, both the replicative and informational functions were carried out by structurally stable β-sheet protein aggregates in a prion-like mode involving templated self-propagation and storage of information in the β-sheet conformation. In this amyloid (protein)-first, hybrid replication-metabolism view, the synthesis of RNA, and the evolvement of an RNA-protein world, were later, but necessary events for further biomolecular evolution to occur. I further argue that in our contemporary DNA↔RNA→protein world, the primordial β-conformation-based information system is preserved in the form of a cytoplasmic epigenetic memory.
Similar content being viewed by others
Introduction
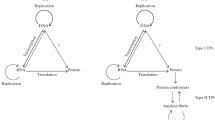
It is generally assumed that life on earth evolved in a sequential way starting with an intial prebiotic chemical synthesis of amino acids and nucleobases for ~3.9 billion years ago. Experimental support for this idea has been obtained by showing that the synthesis of peptides and ribonucleotides can be achieved under conditions simulating those believed to have existed at that time. Given the informational and autocatalytic properties of ribonucleotides, the concept of a primordial RNA world (Gilbert 1986; Joyce 2002; Orgel 2004) has aroused great interest. However, despite its appeal, it has been difficult to explain the emergence of both stable and meaningful RNA molecules under the prevailing harsh conditions of high, near boiling-point of water temperatures, atmospheric electrical discharges and cosmic radiation, all known to decompose polyribonucleotides (Elicieri et al. 1989; Larrade et al. 1995; Levy and Miller 1998; Lindahl 1993).
Though less popular, the peptide/protein-first hypothesis (Ikehara 2005; Rode 1999; Woolfson 2000) has remained an alternative. It is well-known that peptides are readily formed under abiotic conditions. In the presence of a mineral surface peptides up to 55 monomers long can be synthesized (Ferris et al. 1996). Such peptides, as well as shorter, can adopt functional three-dimensional structures and assemble through autocatalysis (Struthers et al. 1996; Lee et al. 1996; Issac et al. (2001). They may, moreover, express both chiroselectivity and perform dynamic error correction (Issac et al. 2001). The peptide/protein model, like the RNA model, is, however, problematic with respect to the stability of the molecules under the extreme conditions on the primitive earth; the native three-dimensional protein structure is likely to decompose under such conditions.
Here I advance the idea that in the prebiotic peptide/protein world, cross β-sheet polypeptide aggregates that, in contrast to natively folded polypeptides, possess a rigid, high temperature-resistant structure and self-propagating characteristics, were the first replicating and information-processing molecules that evolved. In this protein-first, hybrid replication-metabolism view, the synthesis of RNA, and the evolvement an RNA-protein world, were later, but necessary events for further biomolecular evolution to occur. I further argue that in our contemporary DNA↔RNA→protein world, the primordial β-conformation-based information system is preserved in the form of an adaptive cytoplasmic epigenetic memory. My arguments are largely based on recent advancements in prion and amyloid biology and I shall begin with some of the key achievements that have substantially changed our view on protein conformatics and information transfer.
Metamorphic Proteins
For a protein to be biologically active, folding into its native three-dimensional structure is essential. Anfinsen´s principle (Anfinsen 1973) states that a polypeptide achieves its biologically active state by descending to the thermodynamically most favorable conformation corresponding to one of some few thousand possible conformers. New data indicate that this empirical rule may, however, need revision (Boehr and Wright 2008; Murzin 2008): for the same amino acid sequence an increasing number of proteins have been shown to adopt under native conditions various folded conformations that exist in dynamic equilibrium. Moreover, in addition to the production of natively folded protein isomers, dysfolding through partial folding or unfolding may occur (Chiti and Dobson 2006; Jahn and Radford 2008; Kodali and Wetzel 2007; Maury 2009). Such dysfolded intermediates can by a templated seeding/nucleation mechanism give rise to specific, preamyloid polymorphic β-sheet aggregates or mature amyloid fibrils (Chiti and Dobson 2006; Collins et al. 2004; Hamley 2007; Makin and Serpell 2005; Zhang and Muthukumar 2009; Table 1). Importantly, amyloid formation is not always pathological, but may be functional. Beneficial amyloids have been found in a wide range of organisms, from bacteria to mammals with functions as diverse as biofilm formation, development of aerial structures, scaffolding, regulation of melanin synthesis, epigenetic control of polyamines, and information transfer (Gebbink et al. 2005; Fowler et al. 2007; Maury 2009; Namy et al. 2008; Shorter and Lindquist 2005; Wickner et al. 2007). In fact, amyloid formation seems to be a generic propensity of polypeptides and the amyloid β-fold an evolutionary highly conserved primordial structure (Chiti and Dobson 2006; Dobson 2004).
Prions and Amyloids
Prions are self-propagating infectious agents causing a set of devastating neurodegenerative diseases known as the transmissible spongiform encephalopathies (Prusiner 1998). The prion hypothesis posits that a misfolded form of the prion protein, devoid of any nucleic acid, is the causative and transmissible agent in the prion diseases and that the infectivity of the prion particle is related to the conformational state of the protein(Abid and Soto 2006; Prusiner 1998; When-Quan and Cambetti 2005). Notably, the conformational rearrangement of the prion protein results in a stable amyloid structure characterized by a high β-sheet content and high resistance to proteolysis and poor solubility. The propagation of prions is based on a templated self-replicative mechanism very similar to that of amyloids in general (Collins et al. 2004; Serio et al. 2000; Shorter and Lindquist 2004, 2005) The strain specificity of the prions is also related to the specific β-conformational state of the protein. Thus, many of the extraordinary properties of the prions, namely stability, transmissibility, and strain specificity, are dependent on the amyloid fold.
In addition to the infective mammalian prions several naturally occurring functional prions exist. Growing evidence indicates that such prions may be involved in basic cellular functions and encode heritable information. By enhancing the expression of antizyme the yeast prion [PSI+], corresponding to an amyloid conformation of the release factor 3 (Wickner et al. 2007), regulates polyamine metabolism (Namy et al. 2008). The [Het-s] amyloid prion of Podospora anserina, on the other hand, controls heterokaryon formation (Balguerie et al. 2003; Saupe 2000). In yeast, the aggregation and propagation domains of prions are composed of short, low-complexity sequences (Chernoff 2004b). The glutamine/ asparagine (Q/N) rich domains have a particularly high propensity to form self-replicating amyloid and are found both in mesophilic bacteria and eukaryotes (Michelitsch and Weissman 2000). Importantly, a striking homology between the sequences of known prions and peptides formed in the salt-induced peptide formation reaction, simulating early earth conditions, has been observed (Rode et al. 1999).
Protein-Mediated Information Transfer
Growing evidence favours the protein-only hypothesis stating that information encrypted in the dysfolded three-dimensional protein structure is transmittable (Chernoff 2004a; Chiti and Dobson 2006; King and Diaz-Avalos 2004; Prusiner 1998; Ritter et al. 2005; Shorter and Lindquist 2005; Tanaka et al. 2004; When-Quan and Cambetti 2005; Wickner et al. 2008). The parallel in-register β-sheet amyloid structure of fungal prions (Kajava et al. 2004; Wickner et al. 2008) allows self-propagation and transfer of conformational information by a templating mechanism as well as the encoding of heritable traits (Wickner et al. 2008). Synthetic fungal and mammalian prion amyloids induce prion phenotypes in their corresponding hosts (Legname et al. 2004) and a specific 71-mer β strand amyloid entity of the HET-s prion has been shown to be the infectious unit of the HET-s prion (Ritter et al. 2005). The protein-only nature of [PIN+] was recently established by demonstrating a direct correspondence between in vitro produced amyloid-like Rnq1p assemblies and the in vivo replication of [PIN+] prions (Patel and Liebman 2007). In contrast to the fungal prions [PSI+], [URE3], [Het-s] and [PIN+], the protein-only character of mammalian prions have been more difficult to establish. So far, the best evidence has been provided by Legname et al. (2004) showing that a fragment of mouse prion protein after misfolding into β-sheet fibrils can induce prion disease, and by Castilla et al. (2005) and Weber et al. (2006, 2007) demonstrating the infectious nature of scrapie-like prions generated in vitro by a cyclic amplification. However, these studies do not provide definitive proofs for the protein-only nature of mammalian prions; arguments against it include reservations regarding the purity of the infectious protein preparations used (Manuelidis 2006) and the possibility that inoculated mutant misfolded prions only promote disease (Nazor et al. 2005).
The Primordial β-sheet Polypeptide World
To be valid, a theory on the origin of life has to comprise, and be able to explain, at the minimum, the most fundamental attributes of living systems, i.e. the ability to replicate, evolve, increase in complexity and compartmentalize under the extreme conditions prevailing on the early earth. I argue that the β-sheet amyloid model meets these requirements (Table 2).
Amyloid Generation and Stability
In chemical experiments simulating probable early earth conditions, including non-reducing conditions with N2 and CO2, amino acids and peptides are readily formed (Brack 2007; Brack and Orgel 1975; Cleaves et al. 2008; Huber and Wächtershauser 1998; Huber et al. 2003, Imai et al. 1999; Leman et al. 2004; Li et al. 2008; Miller 1953, 1997; Orgel 2004; Rode 1999; Woolfson 2000). In the Miller experiments a large number of various amino acids were generated including significant amounts of hydrophobic residues (Johnson et al. 2008; Miller 1997; Ring et al. 1972). Since amyloid formation is a common propensity of polypeptides in general and almost any amino acid sequence can be amyloidogenic under appropriate conditions (Chiti and Dobson 2006), the prerequisites for prebiotic amyloid formation exist. The relatively high content of hydrophobic residues observed in the randomly produced peptide mixtures, as well as the presence of alternating hydrophobic and hydrophilic residues (Brack 2007; Brack and Orgel 1975) increases the β-sheet forming potential of such mixtures (Dyson et al. 2006; Galitskaya et al. 2006; Maury 1994). Under the harsh prebiotic conditions, the highly stable amyloid structure would have been an obvious selective advantage over the competitive, natively folded structure: the cross β sheet conformation is resistant to both UV and ionizing radiation, and to high temperatures (Alper 1993; Brack 2007; Fernie et al. 2007; Meersman and Dobson 2006). Germicidal UV radiation does not inactivate the amyloid-dependent prion infectivity, nor 30–120 min exposures to temperatures of 100°C or even higher. The β sheet conformation system fulfils, hence, two basic requirements of any prebiotic chemical evolution model: (i) that the structural components can be generated by random processes from compounds present in the presumed primordial environment and (ii) that the components can provide sufficient stability to allow their spreading in that environment.
Replication and Variability
The ability to replicate and evolve is quintessential to all living systems and is a key feature of the current β sheet model, too. Amyloid propagates by a self-templating mechanism in which an intial slow nucleation phase is followed by fast kinetics where monomers/oligomers are added to the growing protofibril (Chiti and Dobson 2006; Jahn and Radford 2008; Serio et al. 2000; Maury 2009; Pellarin et al. 2007; Wang et al. 2008). Importantly, even very short peptides, 3 to 11 monomers long, may self-assemble to amyloid fibrils (Balbirnie et al. 2001; Goux et al. 2004; Madine et al. 2009; Maury 1994; Maury and Nurmiaho-Lassila 1992; Reches et al. 2002; Fig. 1). During amyloidogenesis a varying number of intermediates are formed and for any given sequence several three-dimensional structures may be produced. The conformational variability can then be passed on to new molecular structures in a conformation (strain)—specific manner. The environmentally best fittest variants and those with the fastest over-all production rate are likely to become the most populated structures. The β-conformational system may, in a way, be considered a primeval form of heredity in which information is stored in the β-sheet structure and then transmitted to daughter molecules.
a Characteristic green birefringence of amyloid (Congo-red staining, polarized light). b Amyloid fibrils spontaneously created from a gelsolin-related synthetic peptide (SFNNGYCFILD) containing 45% hydrophobic residues. Experimental conditions were as described (Maury and Nurmiaho-Lassila 1992) (negative staining, electron microscopy ×67000). c Schematic representation of an antiparallel β-sheet structure
Organization and Compartmentalization
To achieve higher complexity, compartmentalisation is essential for all evolving molecular systems (Eigen 1971) and is a feature of the early chemical evolution models of Oparin (Novak 1984), Fox (1980), and Wächtershäuser (1988). With respect to the present model, organization and compartmentalization are key features too. Amyloid possesses the inherent propensity to form self-propagating fibrillar networks that can acts as scaffolds for the amyloid assemblies themselves (Biancalana et al. 2008; Kodama et al. 2004; Krebs et al. 2005; Yagi et al. 2007) as well as for other other molecules such as nucleobases (Nandi and Nicole 2004; Silva et al. 2008) and lipids (Relini et al. 2008; Domano and Kinnunen 2008). In a way similar to that of clay and other mineral beds (Joyce 2002; Franchi et al. 2003) the amyloid surface may also directly enhance the polymerization of polynucleotides (Dale 2006). On the other hand, interfaces, in particular lipid interfaces, can promote amyloid formation from precursor peptides by lowering the activation energy barrier (Relini et al. 2008). The formation of amyloid-based membranes allowing separation of the replicating β-sheet informational molecules from the surrounding medium would have resulted in a certain degree organization and individuality, and, evolvability. Notably, the scaffolding characteristics of amyloid are utilised by several extant organisms: e.g. fish and silk moths use amyloid to protect oocytes from environmental hazards (Iconomidou et al. 2000, Iconomidou and Hamodrakas 2008; Podrabsky et al. 2001 and microbes exploit amyloid as part of a matrix in the process of biofilm formation (Barnhart and Chapman 2006).
Transition to an Amyloid/Protein-RNA World
All theories on the origin of life as well as the question of which biomolecules were the first to populate the primitive earth have remained speculative due to both shortage of knowledge of the early earth conditions and to limitations in our understanding of living entities and life itself.
RNA molecules, based on their catalytic and informational properties, have, anyhow, been proposed to represent the first biomolecules on earth (Copley et al. 2007; Gilbert 1986; Joyce 2002; Orgel 2004), although it is known that polynucleotides rapidly decompose under the (assumed) primordial earth conditions of high-temperatures and cosmic radiation (Elicieri et al. 1989; Larrade et al. 1995; Lindahl 1993; Levy and Miller 1998). The same critique applies to the peptide-first theory: the stability of the native three-dimensional protein structure can, under such conditions, be questioned. The amyloid-first hypothesis advanced herein offers a solution to the stability problem and combines the replication- and metabolism-first paradigms: the structurally stable β-sheet aggregates carry out both the replicative and informational functions in a prion-like mode involving templated self-propagation and storage of adaptive information in transmittable β-conformations. The model fulfils a key necessity of a valid chemical evolution theory too : the ability of the system to evolve. The amyloid-first model does not exclude, but is compatible with a later co-evolving RNA world (Dale 2006; Gilbert 1986; Orgel 2003; Szathmary 1999) which, indeed, was necessary for further biomolecular evolution to occur. The amyloid structure could also have enhanced the polymerization of RNA molecules, as dicussed above, and provided protection for them, as well as for natively folded polypeptides.
The Amyloid Fold in our Contemporary World
There are several examples indicating that the unique properties of the amyloid fold has been conserved in the extant world. Functional amyloids are produced by organisms spanning all aspects of cellular life (Fowler et al. 2007; Maury 2009; Shorter and Lindquist 2005). Bacteria exploit the β-sheet structure for protective purposes. Fungal prion amyloids can constitute molecular memories and transmit epigenetic information. In mammals, amyloids have been shown to regulate melanin biosynthesis, activate hemostatic factors, and even, possibly, in a prion-like mode be involved in long-term memory functions. Obviously, organisms have evolved taking advantage of the canonical β-sheet fold, a conformation that possesses high structural stability and resistance to proteolysis, is self-replicative and has informational properties. On the other hand, amyloid formation must be strictly regulated; deficient control may result in severe pathology, e.g. cerebral and other amyloidoses (Maury 1995; Pettersson et al. 2008). Soluble amyloid oligomers displaying a common conformation are thought to represent the primary pathologic entities in these disorders (Chiti and Dobson 2006, Glabe and Kayed 2006); in some instances toxicity may involve the β-structure only indirectly (Schubert et al. 1995).
Conclusion
I have advanced the idea that the first replicators on the primitive earth were protein-based β-sheet molecular structures that possessed the necessary and sufficient characteristics for making a biomolecular evolution possible under the prevailing conditions; namely, the ability to (i) form stable structures and compartments, (ii) self-propagate and transmit information and, (iii) evolve and increase in complexity. The model represents a hybrid replication-metabolism view and stresses the importance of a later co-evolving and dominating protein-RNA world. In our contemporary DNA↔RNA→protein world, the primordial β-conformation-based information system is suggested to have been preserved in the form of a cytoplasmic epigenetic memory that functions in a prion-like way. The prions—and their amyloid-based functional entities—may, in turn, be considered a relic of an ancient protein-based inheritance system. Finally, the sequential biomolecular evolutionary steps outlined herein, representing the reverse of current translation and transcription processes, may also be considered in the context of the biological principle of “no exception to reversibility” (Zhang 2004).
References
Abid K, Soto C (2006) The intriguing prion disorders. Cell Mol Life Sci 63:2342–2351
Alper T (1993) The scrapie enigma: insights from radiation experiments. Radiat Res 135:283–292
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181:223–230
Balbirnie M, Grothe R, Eisenberg DS (2001) An amyloid-forming peptide from the yeast prion Sup35 reveals a dehydrated beta-sheet structure for amyloid. Proc Natl Acad Sci USA 98:2375–2380
Balguerie A, Dos Reis S, Ritter C, Chaignepain S, Coulary-Salin B et al (2003) Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J 22:2071–2081
Barnhart MM, Chapman MR (2006) Curli biogenesis and function. Annu Rev Microbiol 60:131–147
Biancalana M, Makabe K, Koide A, Koide S (2008) Molecular mechanism of Thioflavine-T binding to the surface of beta-rich peptide assemblies. J Mol Biol Epub Nov 14
Boehr DD, Wright PE (2008) How do proteins interact? Science 320:1429–1430
Brack A (2007) From interstellar amino acids to prebiotic catalytic peptides: a review. Chem Biodivers 4:665–679
Brack A, Orgel LE (1975) Beta structures of alternating polypeptides and their possible prebiotic significance. Nature 256:383–387
Castilla J, Saa P, Hetz C, Soto C (2005) In vitro generation of inectious scrapie prions. Cell 121:195–206
Chernoff YO (2004a) Replication vehicles of protein-based inheritance. Trends Biotechnol 22:549–552
Chernoff YO (2004b) Amyloidogenic domains, prions and structural inheritance: rudiments of early life or recent acquisition? Curr Opin Chem Biol 8:665–671
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid and human disease. Annu Rev Biochem 75:333–366
Cleaves HJ, Chalmers JH, Lazcano A, Miller SL, Bada JL (2008) A reassessment of prebiotic synthesis in neutral planetary atmospheres. Orig Life Evol Biosph 38:105–115
Collins SR, Douglas A, Vale RD, Weissman JS (2004) Mechanism of prion propagation: Amyloid growth occurs by monomer addition. PloS Biology 2:e321
Copley SD, Smith E, Morowitz HJ (2007) The origin of the RNA world: Co-evolution of genes and metabolism. Bioorg Chem 35:430–443
Dale T (2006) Protein and nucleic acid together: a mechanism for the emergence of biological selection. J Theor Biol 240:337–342
Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15:3–16
Domano YA, Kinnunen PK (2008) Islet amyloid polypeptide forms rigid lipid-protein amyloid fibrils on supported phospholipid bilayers. J Mol Biol 376:42–54
Dyson HJ, Wright PE, Scheraga HA (2006) The role of hydrophobic interactions in the initiation and propagation of protein folding. Proc Natl Acad Sci USA 103:13057–13061
Eigen M (1971) Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58:465–523
Elicieri BP, Choudhury K, Scott QO, Elicieri GL (1989) Ultraviolet light-induced inhibition of small nuclear RNA synthesis. J Cell Physiol 138:586–592
Fernie K, Steele PJ, Taylor DM, Somerville RA (2007) Comparative studies of the thermostability of five strains of transmissible-spongiform-enchephalopathy agent. Biotechnol Appl Biochem 47:175–183
Ferris JP, Hill AR Jr, Liu R, Orgel LE (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61
Fowler DM, Koulov AV, Balch WE, Kelly JF (2007) Functional amyloid- from bactetria to humans. Trends Biochem Sci 32:217–224
Fox SW (1980) Metabolic microspheres: origins and evolution. Naturwissenschaften 67:378–383
Franchi M, Ferris JP, Gallorui E (2003) Cations as mediators of the absorption of nucleic acids on clay surfaces in prebiotic environments. Orig Life Evol Biosph 33:1–16
Galitskaya OV, Garbuzynskiy SO, Lobanov MY (2006) Prediction of amyloidogenic and disordered regions in protein chains. PLoS Comput Biol 2:e177
Gebbink MF, Claessen D, Bouma B, Dijkhuizen L, Wösten HA (2005) Amyloids- a functional coat for microorganisms. Nat Rev Microbiol 3:333–341
Glabe CG, Kayed R (2006) Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 66:S74–78
Gilbert W (1986) The RNA World. Nature 319:618
Goux WJ, Kopplin L, Nguyen AD, Leak K, Rutkofsky H, Shanmuganandam VD et al (2004) The formation of straight and twisted filaments from short tau peptides. J Biol Chem 279:26868–26875
Hamley IW (2007) Peptide fibrillization. Angew Chem Int Ed 46:8128–8147
Huber C, Wächtershauser G (1998) Peptides by activation of amino acids with CO on (Ni, Fe) S surfaces: implications for the origin of life. Science 281:670–672
Huber C, Eisenreich W, Hecht S, Wächtershauser G (2003) A possible primordial peptide cycle. Science 301:938–940
Iconomidou VA, Hamodrakas SJK (2008) Natural protective amyloids. Curr Protein Pept Sci 9:291–309
Iconomidou VA, Vriend G, Hamodrakas SJ (2000) Amyloids protect silk moth oocyte and embryo. FEBS Lett 479:141–145
Ikehara K (2005) Possible steps to the emergence of life: The [GADV]-protein world. Chem Rec. 5:107–118
Imai E, Honda H, Hatori K, Brack A, Matsuno K (1999) Elongation of oligopepetides in a stimulated submarine hydrothermal system. Science 283:831–833
Issac R, Ham YW, Chmielewski J (2001) The design of self-replicating helical peptides. Curr Opin Struct Biol 11:458–463
Jahn TR, Radford SE (2008) Folding versus aggregation: Polypeptide conformations on competing pathways. Arch Biochem Biophys 469:100–117
Johnson AP, Cleaves HJ, Dworkin JP, Glavin DP, Lazcano A et al (2008) The Miller volcanic spark discharge experiment. Science 322:404
Joyce GF (2002) The antiquity of RNA-based evolution. Nature 418:214–221
Kajava AV, Baxa U, Wickner RB, Steven AC (2004) A model for Ure2p prion filaments and other amyloids: the parallel superpleated structure. Proc Natl Acad Sci USA 101:7885–7890
King CY, Diaz-Avalos R (2004) Protein-only transmission of three yeast prion strains. Nature 428:319–323
Kodali R, Wetzel R (2007) Polymorphism in the intermediates and products of amyloid assembly. Curr Opin Struct Biol 17:48–57
Kodama H, Matsumura S, Yamashita T, Mihara H (2004) Construction of a protein array on amyloid-like fibrils using co-assembly of designed peptides. Chem Comun 24:2876–2877
Krebs MRH, Bromley EHC, Rogers SS, Donald AM (2005) The mechanism of amyloid spherulite formation by bovine insulin. Biophys J 88:2013–2021
Larrade R, Roberston MP, Miller SL (1995) Rates of decomposition of ribose and other sugars: implications for chemical evolution. Proc Natl Acad Sci USA 92:8158–8160
Lee DH, Granja JR, Martinez JA, Severin K, Ghadri MR (1996) A self-replicating peptide. Nature 382:525–8
Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE et al (2004) Synthetic mammalian proteins. Science 305:673–676
Leman L, Orgel L, Ghadiri MR (2004) Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306:283–286
Levy M, Miller SL (1998) The stability of the RNA bases: Implications for the origin of life. Proc Natl Acad Sci USA 95:7933–7938
Li F, Fitz D, Fraser DG, Rode BM (2008) Methionine peptide formation under primordial earth conditions. J Inorg Biochem 102:12121–1217
Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362:709–715
Madine J, Copland A, Serpell L, Middleton D (2009)Cross-beta spine architecture of fibrils formed by the amyloidogenic segment NFGSVQFV of medin from solid state NMR and X-ray diffraction measurements. Biochemistry, Epub Feb. 5
Makin OS, Serpell LC (2005) Structures for amyloid fibrils. FEBS J 272:5950–5961
Manuelidis L (2006) A 25 nm virion is likely to cause transmissible spongiform enchephalopathies. J Cell Bioch 100:897–915
Maury CPJ (1994) Amyloid fibril formation in gelsolin-derived amyloidosis. Definition of the amyloidogenic region and evidence of accelerated amyloid formation of mutant Asn-187 and Tyr-187 gelsolin peptides. Lab Invest 70:558–564
Maury CPJ (1995) Biology of disease: Molecular pathogenesis of β-amyloidosis in Alzheimer`s disease and other cerebral amyloidoses. Lab Invest 72:4–16
Maury CPJ (2009) The emerging concept of functional amyloid. J Int Med 265:329–334
Maury CPJ, Nurmiaho-Lassila EL (1992) Creation of amyloid fibrils from mutant Asn187 gelsolin peptides. Biochem Biophys Res Commun 183:227–231
Meersman F, Dobson CM (2006) Probing the pressure-temperature stability of amyloid fibrils provides new insights into their molecular properties. Biochim Biophys Acta 1764:452–460
Michelitsch MD, Weissman JS (2000) A census of glutamine/aspargine-rich regions: implications for their conserved function and the prediction of novel prions. Proc Natl Acad Sci USA 97:11910–11915
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117:528–529
Miller SL (1997) Peptide nucleic acids and prebiotic chemistry. Nat Struct Biol 4:167–169
Murzin AG (2008) Metamorphic proteins. Science 320:1725–1726
Namy O, Galopier A, Martini C, Matsufuji S, Fabret C, Roussel JP (2008) Epigenetic control of polyamines by the prion [PSI+]. Nat Cell Biol 10:1069–1075
Nandi PK, Nicole JC (2004) Nucleic acid and prion protein interaction produces spherical amyloids which can function in vivo as coats of spongiform encephalopathy agent. J Mol Biol 344:827–837
Nazor KE, Kuhn F, Seward T, Green M, Zwald D et al (2005) Immunodetection of disease-associated mutant PrP which accelerates disease in GSS transgenic mice. EMBO J 24:2472–2480
Nelson R, Eisenberg D (2006) Recent atomic models of amyloid structure. Curr Opin Struct Biol 16:260–265
Novak VJ (1984) Present state of the coacervate-in-coacervate theory: origin and evolution of the cell structure. Orig Life 14:513–522
Orgel LE (2003) Some consequences of the RNA world hypothesis. Orig Life Evol Biosph 33:211–218
Orgel LE (2004) Prebiotic chemistry and the origin of the RNA World. Crit Rev Biochem Mol Biol 39:99–123
Patel BK, Liebman SW (2007) “Prion-proof” for [PIN+]: Infection with in vitro-made amyloid aggregates of Rnq1p-(132–405) induces [PIN+]. J Mol Biol 3645:773–782
Pellarin R, Guarnera E, Caflisch A (2007) Pathways and intermediates of amyloid fibril formation. J Mol Biol 374:917–924
Pettersson T, Konttinen YT, Maury CPJ (2008) Treatment strategies for amyloid A amyloidosis. Expert Opin Pharmacother 9:2117–2128
Podrabsky JE, Carpenter JF, Hand SC (2001) Survival of water stress in fish embryos: dehydration avoidance and egg envelope amyloid fibers. Am J Physiol Regul Integr Comp Physiol 280:R123–131
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:133363–133383
Reches M, Porat Y, Gazit E (2002) Amyloid fibril formation by pentapeptide and tetrapeptide fragments of human calcitonin. J Biol Chem 277:35475–35480
Relini A, Cavalleri O, Rolandi R, Gliozzi A (2008) The two fold aspect of the interplay of amyloidogenic proteins with lipid membranes. Chem Phys Lipids 158:1–9
Ring D, Wolman Y, Friedmann N, Miller SL (1972) Prebiotic synthesis of hydrophobic and protein amino acids. Proc Natl Acad Sci USA 69:765–768
Ritter C, Maddelein ML, Siemer AB, Luhrs T, Ernst M et al (2005) Correlation of the structure and infectivity of the HET-s prion. Nature 435:844–848
Rode BM (1999) Peptides and the origin of life. Peptides 20:773–786
Rode BM, Flader W, Sotriffer C, Righi A (1999) Are prions a relic of an early stage of peptide evolution? Peptides 20:1513–1516
Saupe SJ (2000) Molecular genetics of heterokaryon incompatibility in filamentous Ascomycetes. Microbiol Mol Biol Rev 64:489–502
Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi J et al (2000) Nucleated conformational conversion of the replication of conformational information by a prion determinant. Science 298:1317–1321
Shorter J, Lindquist S (2004) Hsp 104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science 304:1793–1797
Shorter J, Lindquist S (2005) Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 6:435–450
Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Gagara Y, Kimura H (1995) Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci USA 92:1989–1993
Silva JL, Lima LM, Foguel D, Cordeiro Y (2008) Intriguing nucleic acid-binding features of mammalian prion protein. Trends Biochem Sci 33:132–140
Struthers MD, Cheng RP, Imperiali B (1996) Design of a monomeric 23-residue polypeptide with defined tertiary structure. Scuience 271:342–345
Szathmary E (1999) The origin of the genetic code. Trends Genet 15:223–229
Tanaka M, Chien P, Naber N, Cooke R, Weissman JS (2004) Conformational variation in an infectious prion protein determine prion starin differences. Nature 248:323–328
Wang X, Hammer ND, Chapman MR (2008) The molecular basis of functional bacterial amyloid polymerization and nucleation. J Biol Chem 283:21530–21539
Weber P, Giese A, Piening N, Mittereger G, Thomizig A et al (2006) Cell-free formation of misfolded prion protein with authentic prion infectivity. Proc Natl Acad Sci USA 103:15818–15823
Weber P, Giese A, Piening N, Mitteregger G, Thomzig A et al (2007) Generation of genuine prion infectivity by serial PMCA. Vet Microbiol 123:346–357
When-Quan Z, Cambetti P (2005) From microbes to prions: the final proof of the prion hypothesis. Cell 2005 12:155–162
Wickner RB, Edskes HK, Shewmakeer F, Nakayashiki T (2007) Prions of fungi: inherited structures and biological roles. Nat Rev Microbiol 5:611–617
Wickner RB, Shewmaker F, Kryndushkin D, Edskes HK (2008) Protein inheritance (prions) based on parallel in-register ß-sheet amyloid structures. BioEssays 30:955–964
Woolfson A (2000) Life without genes. Flamingo, London. ISBN 978-0006548744
Wächtershäuser G (1988) Before enzymes and templates: theory of surface metabolism. Microbiol Rev 52:452–484
Yagi H, Ban T, Morigakli K, Naiki H, Goto Y (2007) Visualization and classification of amyloid beta supramolecular assemblies. Biochemistry 46:15009–15017
Zhang Y (2004) Molecular biology: No exception to reversibility. Nature 431:637–639
Zhang J, Muthukumar M (2009) Simulations of nucleation and elongation of amyloid fibrils. J Chem Phys 130:035102
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maury, C.P.J. Self-Propagating β-Sheet Polypeptide Structures as Prebiotic Informational Molecular Entities: The Amyloid World. Orig Life Evol Biosph 39, 141–150 (2009). https://doi.org/10.1007/s11084-009-9165-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-009-9165-6