Abstract
In this short article I discuss the relevance of two aspects of vesicle reactivity that are germane to understand the role of compartments in the origin of early cells. Studies of vesicle self-reproduction indicate that simple vesicles can grow and divide, maintaining inside most of their content and giving rise to a simple autopoietic system. New aspects of vesicle reactivity are also introduced, such as selection and competition processes within vesicle populations, emphasizing the concepts of vesicle diversity, inter-vesicles and vesicles–environment interactions, intended as synthetic analogs of primitive ‘ecological’ processes.
Similar content being viewed by others
Introduction
Along the pathway from inanimate matter to living systems, membranous compartments – somehow similar to synthetic lipid vesicles – played a key role in the emergence of the first living cells (Morowitz 1992). Thanks to their spontaneous formation in water, and to the semi-permeable character of their boundary, such compartments may entrap and concentrate in the inner cavity and in the hydrophobic membrane several molecular species present in the environment. Primitive and discrete vesicle-based bodies, especially when carrying catalytically active biopolymers, could have been at the root of the ancient evolution (Deamer 1997; Szostak et al. 2001; Walde 2006).
However, even if nothing is known on the pathway to the first ‘living’ cells, we can inquire about the requirements of such early cells. Far from being perfect, early cells were somehow able to reproduce themselves with a limited set of macromolecule-carried functions, as well as to self-maintain themselves at expenses of environment. The term ‘minimal cells’ (Luisi 2002) has been used to describe such bodies, meaning ‘cells with minimal functions ensuring life’.
Question 7 on “Early Cells” focuses on the minimal number of genes that must be present in a cell in order to be alive, according to a definition of minimal life. A possible answer to question 7 might derive from the construction in the laboratory of minimal cells, which can be then studied and their behavior verified (Luisi et al. 2006). Ideally, the final goal of such approach consists in the definition of the minimal number of components that give rise to a living cell. Together with theoretical studies on the logic of cellular life and minimal functions, to date, a number of reports focus on the use of lipid vesicles as bioreactors, with the aim of realizing simple cell-like structures with limited (and essential) functionalities. Researchers have reported on vesicle bioreactors with one or two working genes, paving the way to the construction of semi-synthetic minimal cells (Luisi et al. 2006).
On the other hand, the studies on nude lipid vesicles, carrying no genes or proteins at all, have also revealed interesting features.
Despite the lack of macromolecule-carried functions, vesicles themselves show a rich landscape of behavior which could have had relevance in the origin of living cells. The aim of this article is to show some of the most intriguing discoveries in such field, and suggesting that some basic vesicle dynamics and reactivity do not need any genetic coded functions. We show that even in the absence of macromolecules, vesicles reactivity allows the achievement of cell-like patterns, as self-reproduction, selection among a population, or competition.
To be in constant interaction with each other and with the environment, a peculiar characteristic of living things, it is also evident in vesicle (eco)systems; the vesicle model is now facing a kind of shift, that explicitly takes into account diversity.
Vesicles Self-Reproduction and the ‘Matrix Effect’
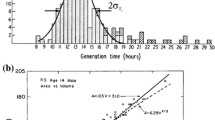
The first interesting issue in vesicle reactivity was the discovery of vesicle self-reproduction. Inspired by previous results with reverse micelles and micelles, Luisi and coworkers (Walde et al. 1994) developed a simple experimental setup where oleate vesicles form thanks to the alkaline hydrolysis of a precursor (oleic anhydride, OA). When OA is stratified over an alkaline solution (bicine buffer, pH 8.5), the hydrolysis can proceed only at the interface between the two liquids. Therefore, the reaction – which produces a mixture of oleic acid and oleate molecules – proceeds slowly at the beginning. As soon as the product concentration reaches the c.v.c. (critical vesicle concentration), oleic acid/oleate vesicles (OV) form spontaneously, due to self-assembly properties of such surfactants. At this point, the system consists of vesicles in the presence of a vesicle precursor (OA). The turbidity–time profile of the reaction is shown in Fig. 1a (curve a), where the sigmodial growth suggests that OA hydrolysis is catalyzed by OV. Interestingly, if pre-formed OV are added to the buffer at the beginning of the reaction, the lag phase disappears, and the plateau corresponding to the OA consumption is quickly reached (curve b). Clearly, the presence of pre-formed OV affects the formation of new vesicles. A sort of ‘vesicle catalysis’ takes place, since OV can uptake fresh OA molecules in the bilayers, where they are hydrolyzed to form the components of the bilayer itself, therefore increasing the total interface. This originates an autocatalytic process. In addition, size analysis reveals a more intriguing aspect. Since the OA hydrolysis leads to an increase of bilayer interface, two different reaction pathways can be theoretically depicted. In the first case, pre-formed (or initially formed) OV grow in size, by enlargement of their membranes and simultaneous solvent uptake; in the second case, growth can be followed by a division event, originated either by vesicle destabilization or by incoherent surface-to-volume growth. Figure 1b shows the size distribution of pre-formed OV before and after the addition of OA. The vesicles, having an initial mean size of 41 nm, after OA uptake and hydrolysis show a mean size of 52 nm, which suggests that vesicle ‘pure growth’ does not take place in the conditions of the experiment (for a pure growth, the final size of 62 nm is expected). Conversely, these data indicates the presence of an autocatalytic process of growth-division, corresponding to vesicle self-reproduction. Notice that the size distribution of new vesicles closely resemble the initial size distribution. Later on this effect was dubbed ‘matrix effect’ to emphasize the effect exerted by pre-existing vesicles on the formation of new vesicles. Since the size does not increase, in addition to vesicle growth, a division process must be considered.
Self-reproduction of oleate vesicles. a Kinetic course of oleic anhydride (0.25 mmol) hydrolysis at 40°C in bicine buffer (200 mM, pH 8.5); in the absence of pre-formed oleate vesicles (curve a); in the presence of pre-formed oleate vesicles (0.20 mmol, curve b). b Vesicle size distribution as measured by electromicrographs analysis; light gray bars: pre-formed 100-nm extruded vesicles before reaction, average diameter 41 nm; dark gray bars: final vesicle size distribution, average diameter 52 nm. Adapted from Walde et al. (1994)
The early observations were done with the system oleate vesicles/oleic anhydride, which, being heterogeneous, needs rigorous experimental arrangement in order to achieve reproducibility. Oleic anhydride can be substituted by soluble oleate micelles, which are converted into oleate vesicles by simple dilution in weakly basic buffer (pH 7.3–9). The resultant homogeneous system (oleate vesicles/oleate micelles) has been deeply investigated, by using turbidity (Bloechliger et al. 1998; Lonchin et al. 1999), dynamic light scattering (Rasi et al. 2003), fluorescence (Chen and Szostak 2004) and by using ferritin-containing vesicles and cryo-transmission electron microscopy in order to study the mechanism of the matrix effect (Berclaz et al. 2001a, b). Recently, a possible intermediate that supports the growth-division pathway was revealed by freeze-fracture electron microscopy; it consists in a sort of dividing vesicle (Stano et al. 2006).
Spontaneous Selection and Competition Processes within a Vesicle Population
In recent years, a small group of publications reported on spontaneous selection and competition processes within a vesicle population. These two issues represent the newest aspect of vesicle population studies, and probably will be developed further in the future within the paradigm of systems-oriented approaches. The relevance of these studies stems from the great importance of physicochemical processes in the very early stages of biochemical evolution, when compartment protocells experienced selective pressure and competition. Few reports explicitly refer to selectivity within a vesicle population, and even more meager is the current literature on vesicle competition, with the notable exceptions described below.
Vesicle populations often display heterogeneity, and sub-populations can be selected thanks to an appropriate factor. For example, when, at 10°C, vesicles composed by (R)- or (S)-2-methyldodecanoic acid are fed with (S)-2-methyldodecanoic anhydride, (R)-type vesicles slowly transform into ramemic vesicles and precipitate, whereas (S)-type vesicles remain homochiral and stay in suspension (Morigaki et al. 1997). In this case the joint effects of an external constrain (the temperature) and the chirality of the precursor selects homochiral vesicles. This selection mechanism would powerfully select homochiral vesicles in appropriate conditions.
Vesicle morphology can also be discriminated by peculiar interactions: whereas unilamellar lecithin vesicles are substantially unaffected by Trp oligopeptides (W n), large multilamellar vesicles and vesicle clusters promptly react with W 2 and W 3 to give smaller and soluble particles (Stano et al. 2005). Although its mechanism is still unknown, this peptides–vesicles interaction may lead to selective fission/rearrangement pathways.
Recently, Thomas and Luisi (2005) have shown that positively doped lecithin vesicles (containing 3.5% cetyltrimethylammonium bromide) selectively interact with tRNA to give: (1) a stable suspension, or (2) bridged vesicle aggregates, depending on the vesicles size. Figure 2a shows that large unilamellar vesicles (diameter 160 nm) readily aggregate in the presence of tRNA, whereas smaller vesicles (80 nm) do not. In addition, it has been shown that a mixture of small and large unilamellar cationic vesicles can undergo spontaneous selection in the presence of tRNA, which removes from solution the larger vesicles without affecting the smaller vesicles (Fig. 2b). Experiments with very heterogeneous vesicle populations (50–500 nm) show that after the selection only vesicles with size below ca. 100 nm can stay in solution. In conclusion, the RNA-mediated selection of vesicles occurs spontaneously within a population of heterogeneous vesicles. Although spontaneous RNA entrapment in these conditions has not been investigated yet, the effect is quite intriguing, especially if one considers that such work makes evident how the ‘RNA World’ scenario and the compartments approach can originate unexpected behavior.
Selective interaction between weakly cationic lecithin vesicles (1-palmitoyl-2-oleoyl-sn-glycero-3-phospocholine 96.5%, cetyltrimethylammonium bromide 3.5%) and tRNA (from E. coli). a Turbidity profile of 160 nm vesicles (a) and 80 nm vesicles (b) after the addition of excess tRNA. No turbidity changes can be recorded for the smaller vesicles, whereas larger vesicle readily undergo tRNA-mediated aggregation with strong turbidity increase. Dynamic light scattering analysis of final sample show that large particles (>1 μm) have been formed. b When tRNA is added to a vesicle population containing small and large vesicles, small vesicles remain unaffected, whereas large aggregates separate from the solution. Binding of tRNA to both small and large vesicles has been confirmed by zeta potential measurement. Data taken from Thomas and Luisi (2005)
Competition processes have been investigated only recently. The first report is provided by Cheng and Luisi (2003), who determined that the rate of oleate vesicle growth by incorporation of a membranogenic precursor (oleate micelles) does depends on vesicle size. In fact, larger vesicles (diameter: 130 nm) have higher attitude to undergo self-reproduction when compared with smaller vesicles (65 nm), as judged by comparing turbidity vs. time plots (t 1/2 = 135 and 390 s, respectively). Therefore, in a population of vesicles having different sizes, it is expected a competition between vesicles for membrane precursor.
The second case has been reported by Chen et al. (2004) and concerns competition between vesicles that differ for their osmotic state. In particular, it has been shown that encapsulated RNA exerts an osmotic pressure on vesicle membrane with the result that RNA-containing vesicles can grow at the expenses of osmotically relaxed (isotonic) vesicles, which shrink. This phenomenon might provide functional advantage for those compartments on the basis of their content.
Selection and competition processes are particularly relevant in the early cells or protocells scenario, especially with the aim of discovering how primitive biopolymers or simple physical forces could have been involved in selecting and modifying primitive compartments dynamics. From the data shown above, selection can be achieved by probing properties related to membrane fluidity, vesicle morphology, and size; competition has been investigated between vesicles with different size or different osmotic state.
Moreover, the theoretical works of Lancet (Segré and Lancet 2000), Svetina (Bozic and Svetina 2004) and stochastic simulations carried out by Mavelli (Mavelli and Ruiz-Mirazo 2007) are also related to ‘vesicle diversity’ and the associated mechanisms of selection introduced in this short article.
This new research area is at its infancy, but is rather intriguing and promising. The concepts of selection and competition among vesicle populations and the largely unexplored landscape of vesicle reactivity should be probed, possibly providing more adherent and informative models for compartments roles in origins of life studies.
References
Berclaz N, Mueller M, Walde P, Luisi PL (2001a) Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J Phys Chem B 105:1056–1064
Berclaz N, Bloechliger E, Mueller M, Luisi PL (2001b) Matrix effect of vesicle formation as investigated by cryotransmission electron microscopy. J Phys Chem B 105:1065–1071
Bloechliger E, Blocher M, Walde P, Luisi PL (1998) Matrix effect in the size distribution of fatty acid vesicles. J Phys Chem 102:10383–10390
Bozic B, Svetina S (2004) A relationship between membrane properties forms the basis of a selectivity mechanism for vesicle self-reproduction. Eur Biophys J 33:565–571
Chen I, Szostak JW (2004) A kinetic study of the growth of fatty acid vesicles. Biophys J 87:988–998
Chen IA, Roberts RW, Szostak JW (2004) The emergence of competition between model protocells. Science 305:1474–1476
Cheng Z, Luisi PL (2003) Coexistence and mutual competition of vesicles with different size distributions. J Phys Chem B 107:10940–10945
Deamer DW (1997) The first living systems: a bioenergetic perspective. Microbiol Mol Biol Rev 61:239–261
Lonchin S, Luisi PL, Walde P, Robinson BH (1999) A matrix effect in mixed phospholipid/fatty acid vesicle formation. J Phys Chem B 103:10910–10916
Luisi PL (2002) Toward the engineering of minimal living cells. Anatom Rec 268:208–214
Luisi PL, Ferri F, Stano P (2006) Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 93:1–13
Mavelli F, Ruiz-Mirazo K (2007) A stochastic simulation model of minimal self-(re-)producing cellular systems. Philos Trans R Soc (in press)
Morigaki K, Dallavalle S, Walde P, Colonna S, Luisi PL (1997) Autopoietic self-reproduction of chiral fatty acid vesicles. J Am Chem Soc 119:292–301
Morowitz HJ (1992) Beginnings of cellular life. Metabolism recapitulates biogenesis. Yale University Press, New Haven
Rasi S, Mavelli F, Luisi PL (2003) Cooperative micelle binding and matrix effect in oleate vesicle formation. J Phys Chem B 107:14068–14076
Segré D, Lancet D (2000) Composing life. EMBO Rep 1:217–222
Stano P, Bufali S, Domazou AS, Luisi PL (2005) Effect of tryptophan oligopeptides on the size distribution of POPC liposomes: a dynamic light scattering and turbidimetric study. J Lipos Res 15:29–47
Stano P, Wehrli E, Luisi PL (2006) Insights on the oleate vesicles self-reproduction. J Phys Condens Matter 18:S2231–S2238
Szostak JW, Bartel DP, Luisi PL (2001) Synthesizing life. Nature 409:387–390
Thomas CF, Luisi PL (2005) RNA selectively interacts with vesicles depending on their size. J Phys Chem B 109:14544–14550
Walde P (2006) Surfactant assemblies and their various possible roles for the origin(s) of life. Orig Life Evol Biosph 36(2):109–150
Walde P, Wick R, Fresta A, Mangone A, Luisi PL (1994) Autopoietic self-reproduction of fatty acid vesicles. J Am Chem Soc 116:11649–11654
Acknowledgements
I thank Prof. Pier Luigi Luisi, Dr. Chris Thomas (University of Roma3) and Dr. Zhilang Cheng (University of Arizona) for stimulating discussions. As co-organizer of the Erice School, I am grateful to Dr. Fiorella Ruggiu (Ettore Majorana Center, Erice), for her precious help in the organization of the event.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stano, P. Question 7: New Aspects of Interactions Among Vesicles. Orig Life Evol Biosph 37, 439–444 (2007). https://doi.org/10.1007/s11084-007-9086-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-007-9086-1