Abstract
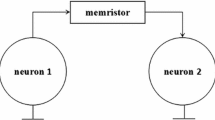
The change of firing rates depends on how synaptic features interact with intrinsic properties of cells in neural system. Considering the chemical synaptic features, we design a controllable memristive synapse with magnetic coupling that are voltage-controlled, nonlinear, and unidirectional. To explore the effect of firing rates on interactions between synapse and neuron, the memristive synaptic current involving excitation and inhibition is then mapped into a generalized neuronal model. We observe and characterize the appearance of counterintuitive behavior that increased excitatory memristive synaptic current leads to the decrease in firing rates, and increased inhibitory memristive synaptic current leads to the increase in firing rates in the neural circuit. For the counterintuitive phenomenon, we utilize a geometric dynamics method to provide an underlying dynamics mechanism how the excitatory or inhibitory current impacts the decrease or increase in firing rates.











Similar content being viewed by others
References
Denève, S., Machens, C.K.: Efficient codes and balanced networks. Nat. Neurosci. 19, 375–382 (2016)
Pecka, M., Han, Y., Sader, E., Mrsic-Flogel, T.D.: Experience-dependent specialization of receptive field surround for selective coding of natural scenes. Neuron. 84, 457–469 (2014)
Sun, Y.J., Wu, G.K., Liu, B.H., Li, P., Zhou, M., Xiao, Z., Tao, H.W., Zhang, L.I.: Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature 465, 927–931 (2010)
Hodgkin, A.L., Huxley, A.F.: Action potentials recorded a Mauthner from inside. Nature 144, 710–711 (1939)
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
Hodgkin, A.L.: The local electric changes associated with repetitive action in a non-medullated axon. J. Physiol. 107, 165–181 (1948)
Prescott, S.A., De Koninck, Y., Sejnowski, T.J.: Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput. Biol. 4, e1000198 (2008)
Perkel, D.H., Schulman, J.H., Bullock, T.H., Moore, G.P., Segundo, J.P.: Pacemaker neurons: effects of regularly spaced synaptic input. Science 145, 61–63 (1964)
Kuffler, S.W., Eyzaguirre, C.: Synaptic inhibition in an isolated nerve cell. J. Gen. Physiol. 39, 155–184 (1955)
Park, T.J., Grothe, B., Pollak, G.D., Schuller, G., Koch, U.: Neural delays shape selectivity to interaural intensity differences in the lateral superior olive. J. Neurosci. 16, 6554–6566 (1996)
Sanes, D.: An in vitro analysis of sound localization mechanisms in the gerbil lateral superior olive. J. Neurosci. 10, 3494–3506 (1990)
Chance, F.S., Abbott, L.F., Reyes, A.D.: Gain modulation from background synaptic input. Neuron. 35, 773–782 (2002)
Zador, A.M., Stevens, C.F.: Input synchrony and the irregular firing of cortical neurons. Nat. Neurosci. 1, 210–217 (1998)
Mitchell, S.J., Silver, R.A.: Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 38, 433–445 (2003)
Carvalho, T.P., Buonomano, D.V.: Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input–output functions. Neuron 61, 774–785 (2009)
Harsch, A., Robinson, H.P.C.: Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance. J. Neurosci. 20, 6181–6192 (2000)
Dodla, R., Svirskis, G., Rinzel, J.: Well-timed, brief inhibition can promote spiking: postinhibitory facilitation. J. Neurophysiol. 95, 2664–2677 (2006)
Beiderbeck, B., Myoga, M.H., Müller, N.I.C., Callan, A.R., Friauf, E., Grothe, B., Pecka, M.: Precisely timed inhibition facilitates action potential firing for spatial coding in the auditory brainstem. Nat. Commun. 9, 1771 (2018)
Kopp-Scheinpflug, C., Sinclair, J.L., Linden, J.F.: When sound stops: offset responses in the auditory system. Trends Neurosci. 41, 712–728 (2018)
Myoga, M.H., Lehnert, S., Leibold, C., Felmy, F., Grothe, B.: Glycinergic inhibition tunes coincidence detection in the auditory brainstem. Nat. Commun. 5, 1–13 (2014)
Kotak, V.C., Korada, S., Schwartz, I.R., Sanes, D.H.: A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 18, 4646–4655 (1998)
Bacci, A., Huguenard, J.R., Prince, D.A.: Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J. Neurosci. 23, 859–866 (2003)
Perkel, D.H., Mulloney, B., Budelli, R.W.: Quantitative methods for predicting neuronal behavior. Neuroscience 6, 823–837 (1981)
Li, Y., Schmid, G., Hänggi, P., Schimansky-Geier, L.: Spontaneous spiking in an autaptic Hodgkin–Huxley setup. Phys. Rev. E 82, 061907 (2010)
Wang, H., Chen, Y.: Influence of autapse on mode-locking structure of a Hodgkin–Huxley neuron under sinusoidal stimulus. J. Theor. Biol. 358, 25–30 (2014)
Yilmaz, E., Ozer, M., Baysal, V., Perc, M.: Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci. Rep. 6, 30914 (2016)
Baysal, V., Yilmaz, E., Özer, M.: Blocking of weak signal propagation via autaptic transmission in scale-free networks. Istanb. Univ. J. Electr. Electron. Eng. 17, 3081–3085 (2017)
Yilmaz, E., Baysal, V., Ozer, M., Perc, M.: Autaptic pacemaker mediated propagation of weak rhythmic activity across small-world neuronal networks. Phys. A. 444, 538–546 (2016)
Bera, B.K., Ghosh, D., Lakshmanan, M.: Chimera states in bursting neurons. Phys. Rev. E. 93, 012205 (2016)
Majhi, S., Ghosh, D.: Alternating chimeras in networks of ephaptically coupled bursting neurons. Chaos 28, 083113 (2018)
Bera, B.K., Rakshit, S., Ghosh, D., Kurths, J.: Spike chimera states and firing regularities in neuronal hypernetworks. Chaos 29, 053115 (2019)
Majhi, S., Perc, M., Ghosh, D.: Chimera states in uncoupled neurons induced by a multilayer structure. Sci. Rep. 6, 39033 (2016)
Gjoni, E., Zenke, F., Bouhours, B., Schneggenburger, R.: Specific synaptic input strengths determine the computational properties of excitation-inhibition integration in a sound localization circuit. J. Physiol. 596, 4945–4967 (2018)
Gerstner, W., Kistler, W., Naud, R., Paninski, L.: Neuronal Dynamics From Single Neurons to Networks and Models of Cognition. Cambridge University Press, Cambridge (2014)
Markram, H., Wang, Y., Tsodyks, M.: Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. 95, 5323–5328 (2002)
Brughera, A.R., Stutman, E.R., Carney, L.H., Colburn, H.S.: A model with excitation and inhibition for cells in the medial superior olive. Audit. Neurosci. 2, 219–233 (1996)
Large, E.W., Crawford, J.D.: Auditory temporal computation: Interval selectivity based on post-inhibitory rebound. J. Comput. Neurosci. 13, 125–142 (2002)
Brand, A., Behrend, O., Marquardt, T., McAlpine, D., Grothe, B.: Precise inhibition is essential for microsecond interaural time difference coding. Nature 417, 543–547 (2002)
Li, X., Jia, Y., Wang, Y., Kui, Z.: The collective bursting dynamics in a modular neuronal network with synaptic plasticity. Nonlinear Dyn. 89, 2593–2602 (2017)
Rothman, J.S., Young, E.D., Manis, P.B.: Convergence of auditory nerve fibers onto bushy cells in the ventral cochlear nucleus: implications of a computational model. J. Neurophysiol. 70, 2562–2583 (1993)
Casadiego, J., Maoutsa, D., Timme, M.: Inferring network connectivity from event timing patterns. Phys. Rev. Lett. 121, 054101 (2018)
Roxin, A., Riecke, H., Solla, S.A.: Self-sustained activity in a small-world network of excitable neurons. Phys. Rev. Lett. 92, 198101 (2004)
Yaroslav, F., Kossio, K., Goedeke, S., van den Akker, B.: Growing critical?: self-organized criticality in a developing neural system. Phys. Rev. Lett. 121, 058301 (2018)
Jonas, P., Racca, C., Sakmann, B., Seeburg, P.H., Monyer, H.: Differences in \(Ca^{2+}\) permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12, 1281–1289 (1994)
Koh, D.S., Geiger, J.R., Jonas, P., Sakmann, B.: \({\rm Ca}^{2+}\)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J. Physiol. 485, 383–402 (1995)
Jahr, C.E., Stevens, C.F.: Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J. Neurosci. 10, 3178–3182 (1990)
Wang, X.-J.: Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J. Neurosci. 19, 9587–9603 (1999)
Higo, S., Akashi, K., Sakimura, K., Tamamaki, N.: Subtypes of GABAergic neurons project axons in the neocortex. Front. Neuroanat. 3, 25–30 (2009)
Braestrup, C., Nielsen, M.: Strychnine as a potent inhibitor of the brain GABA/benzodiazepine receptor complex. Brain Res. Bull. 5, 681–684 (1980)
Zhang, Z., Li, T., Wu, Y., et al.: Truly concomitant and independently expressed short- and long-term plasticity in a \({\rm Bi}_{2}{\rm O}_{2}\)Se-based three-terminal memristor. Adv. Mater. 31, 1805769 (2019)
Najem, J.S., Taylor, G.J., Weiss, R.J., et al.: Memristive Ion channel-doped biomembranes as synaptic mimics. ACS Nano 12, 4702–4711 (2018)
Jo, S.H., Chang, T., Ebong, I., Bhadviya, B.B., Mazumder, P., Lu, W.: Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett. 10, 1297–1301 (2010)
Flores, E.A., Tsang, K.K., Crowell, J.A., Yi, W., Lam, S.K., Bai, X.: Biological plausibility and stochasticity in scalable \({\rm VO}_{{2}}\) active memristor neurons. Nat. Commun. 9, 4661 (2018)
Babacan, Y., Kaçar, F., Gürkan, K.: A spiking and bursting neuron circuit based on memristor. Neurocomputing 203, 86–91 (2016)
Zhang, T., Yang, K., Xu, X., Cai, Y., Yang, Y., Huang, R.: Memristive devices and networks for brain-inspired computing. Phys. Status Solidi Rapid Res. Lett. 13, 1900029 (2019)
Kana, L.K., Fomethe, A., Fotsin, H.B., Wembe, E.T., Moukengue, A.I.: Complex dynamics and synchronization in a system of magnetically coupled colpitts oscillators. J. Nonlinear Dyn. 2017, 5483965 (2017)
Blaha, K.A., Huang, K., Della Rossa, F., Pecora, L., Hossein-Zadeh, M., Sorrentino, F.: Cluster synchronization in multilayer networks: a fully analog experiment with LC oscillators with physically dissimilar coupling. Phys. Rev. Lett. 122, 014101 (2019)
Chua, L.: Memristor-the missing circuit element. IEEE Trans. Circuit Theory 18, 507–519 (1971)
Chua, L.O., Kang, S.M.: Memristive devices and systems. Proc. IEEE 64, 209–223 (1976)
Rose, J.L., Hindmarsh, R.M.R.: A model of neuronal bursting using three coupled first order differential equations. Proc. R. Soc. Lond. B 221, 87–102 (1984)
Rinzel, J.: A Formal Classification of Bursting Mechanisms in Excitable Systems, pp. 267–281. Springer, Berlin (1987)
Barrio, R., Shilnikov, A.: Parameter-sweeping techniques for temporal dynamics of neuronal systems: case study of Hindmarsh–Rose model. J. Math. Neurosci. 1, 6 (2011)
Pereda, A.E.: Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 15, 250–263 (2014)
Rinzel, J.: Bursting Oscillations in an Excitable Membrane Model, pp. 304–316. Springer, Berlin (1985)
Guckenheimer, J., Harris-Warrick, R., Peck, J., Willms, A.: Bifurcation, bursting, and spike frequency adaptation. J. Comput. Neurosci. 4, 257–277 (1997)
Ermentrout, G.B., Terman, D.H.: Mathematical Foundations of Neuroscience, vol. 35. Springer, Berlin (2010)
Sayedaghaee, S.O., Xu, B., Prosandeev, S., Paillard, C., Bellaiche, L.: Novel dynamical magnetoelectric effects in multiferroic \({\rm BiFeO}_{{3}}\). Phys. Rev. Lett. 122, 097601 (2019)
Ma, J., Wu, F., Ren, G., Tang, J.: A class of initials-dependent dynamical systems. Appl. Math. Comput. 298, 65–76 (2017)
Wu, F., Ma, J., Ren, G.: Synchronization stability between initial-dependent oscillators with periodical and chaotic oscillation. J. Zhejiang Univ. A. 19, 889–903 (2018)
Acknowledgements
The authors thank editor Jun Ma and anonymous reviewers for their valuable comments and suggestions that helped to improve the paper. This work is supported by National Natural Science Foundation of China under Grants. 11765011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Model description in phasor domain
Appendix: Model description in phasor domain
Here, we design a controllable memristive system composed of the voltage follow, magnetic coupling, and two multipliers. The schematic diagram is shown in Fig. 12.
The analog circuit composed of two voltage follows (U1, U2), the transformer (T2) to characterize magnetic coupling, and two multipliers (A1, A2). The two red oblique 8-type curves are plotted via analog circuit and numerical stimulation, respectively. Note that the red arrow denotes input voltage and the black arrow denotes output current. (Color figure online)
Note that, our designed memristive system is a voltage-controlled and time-varying devices with only a unidirectional output current \(i_{M}\). This system also exhibits current–voltage hysteresis in the inset of Fig. 12. Applying the Kirchhoff’s law into the magnetic coupling part of circuit mentioned above, the coupling equations are obtained by
where \(i_{1}\), \(V_{1}\), and \(i_{2}\), \(V_{2}\) represent the time-harmonic current and voltage in primary coil and secondary coil, respectively. The voltage follow U1 can guarantee the unidirectional current input, and \(V_{1}\) is equal to \(V_{\mathrm{pre}}\). R, L, and M are the resistance, self-inductance, and mutual inductance. \(j\omega \) denotes the derivative with respect to time variable \(\tau \). \(\omega \) is the angular frequency. Due to the existence of a voltage follow U2, the current in secondary circuit is zero as \(i_{2}= 0\). Thus, Eq. (S1) is rewritten as
where \(R_{1}\) is the controllable resistance to mediate the oscillatory frequency so that there is a different relationship between current and voltage in the whole circuit. Through the analog multiplier A1, we get
The eventual output signal in the phasor-domain representation through the analog multipliers A2 reads
The eventual output current in the time-domain representation reads
Further, the potential scale transformation is considered as following equations
Substituting Eq. (S6) into Eq. (S5), the memristive synaptic current is given by
where \(i_{M}\) denotes the output current through a memristive system involving magnetic coupling and w is a dimensionless internal state. The approach based on the phasor domain is also able to describe the memristive synaptic current of Eq. (S7) consistent with Eq. (9) obtained from the time domain.
Rights and permissions
About this article
Cite this article
Wu, F., Zhang, Y. & Zhang, X. Regulating firing rates in a neural circuit by activating memristive synapse with magnetic coupling. Nonlinear Dyn 98, 971–984 (2019). https://doi.org/10.1007/s11071-019-05239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-019-05239-4