Abstract
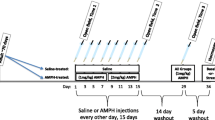
Prenatal exposure to amphetamine induces changes in dopamine receptors in mesolimbic areas and alters locomotor response to amphetamine during adulthood. Sex differences have been reported in amphetamine-induced brain activity and stress sensitivity. We evaluated the effects of prenatal amphetamine exposure on locomotor activity, dopamine receptors and tyrosine hydroxylase mRNA expression in nucleus accumbens and caudate-putamen in response to amphetamine challenge in adult female and male rats. The role of estrogen in the response to restraint stress was analyzed in ovariectomized, prenatally amphetamine-exposed rats. Pregnant rats were treated with d-amphetamine during days 15–21 of gestation. Nucleus accumbens and caudate-putamen were processed for mRNA determination by real-time PCR. In nucleus accumbens, higher mRNA dopamine (D3) receptor expression was found in basal and d-amphetamine-challenge conditions in female than male, and prenatal amphetamine increased the difference. No sex differences were observed in caudate-putamen. Basal saline-treated females showed higher locomotor activity than males. Amphetamine challenge in prenatally amphetamine-exposed rats increased locomotor activity in males and reduced it in females. In nucleus accumbens, estrogen diminished mRNA D1, D2 and D3 receptor expression in basal, and D1 and D3 in ovariectomized stressed rats. Estrogen prevented the increase in tyrosine hydroxylase expression induced by stress in ovariectomized prenatally exposed rats. In conclusion, estrogen modulates mRNA levels of D1, D2 and D3 receptors and tyrosine hydroxylase expression in nucleus accumbens; prenatal amphetamine-exposure effects on D3 receptors and behavioral responses were gender dependent.






Similar content being viewed by others
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included in the paper.
Abbreviations
- PEA:
-
Prenatal exposure to amphetamine
- CPu:
-
Caudate-putamen
- DA:
-
Dopamine
- d-AMPH:
-
D-amphetamine
- E2:
-
Estradiol
- G15-21:
-
Day15-21 pregnancy
- D1, D2, D3:
-
Dopamine receptors
- NAcc:
-
Nucleus accumbens
- OVX:
-
Ovariectomy
- PND:
-
Postnatal day
- TH:
-
Tyrosine hydroxylase
- VTA:
-
Ventral tegmental area
References
Purves-Tyson TD, Boerrigter D, Allen K, Zavitsanou K, Karl T, Djunaidi V, Double KL, Desai R, Handelsman DJ, Weickert CS (2015) Testosterone attenuates and the selective estrogen receptor modulator, raloxifene, potentiates amphetamine-induced locomotion in male rats. Horm Behav 70:73–84
Mattson BJ, Crombag HS, Mitchell T, Simmons DE, Kreuter JD, Morales M, Hope BT (2007) Repeated amphetamine administration outside the home cage enhances drug-induced Fos expression in rat nucleus accumbens. Behav Brain Res 185(2):88–98
Paz MC, Marchese NA, Cancela LM, Bregonzio C (2013) Angiotensin II AT(1) receptors are involved in neuronal activation induced by amphetamine in a two-injection protocol. Biomed Res Int 2013:534817
Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ (2006) Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci 24(3):867–875
Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ (1994) Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13(5):1235–1244
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64(2):477–505
Rotllant D, Marquez C, Nadal R, Armario A (2010) The brain pattern of c-fos induction by two doses of amphetamine suggests different brain processing pathways and minor contribution of behavioural traits. Neuroscience 168(3):691–705
Hruba L, Schutova B, Slamberova R (2012) Sex differences in anxiety-like behavior and locomotor activity following prenatal and postnatal methamphetamine exposure in adult rats. Physiol Behav 105(2):364–370
Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menendez-Delmestre R, Puig-Ramos A, Torres-Diaz YM (2010) Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav 58(1):33–43
Slamberova R, Macuchova E, Nohejlova K, Stofkova A, Jurcovicova J (2014) Effect of amphetamine on adult male and female rats prenatally exposed to methamphetamine. Prague Med Rep 115(1–2):43–59
Pennacchio GE, Neira FJ, Soaje M, Jahn GA, Valdez SR (2017) Effect of hyperthyroidism on circulating prolactin and hypothalamic expression of tyrosine hydroxylase, prolactin signaling cascade members and estrogen and progesterone receptors during late pregnancy and lactation in the rat. Mol Cell Endocrinol 442:40–50
Slamberova R, Yamamotova A, Pometlova M, Schutova B, Hruba L, Nohejlova-Deykun K, Nova E, Macuchova E (2012) Does prenatal methamphetamine exposure induce cross-sensitization to cocaine and morphine in adult male rats? Prague Med Rep 113(3):189–205
Casarsa BS, Marinzalda MA, Marchese NA, Paz MC, Vivas L, Baiardi G, Bregonzio C (2015) A previous history of repeated amphetamine exposure modifies brain angiotensin II AT1 receptor functionality. Neuroscience 307:1–13
Marchese NA, Occhieppo VB, Basmadjian OM, Casarsa BS, Baiardi G, Bregonzio C (2020) Angiotensin II modulates amphetamine-induced glial and brain vascular responses, and attention deficit via angiotensin type 1 receptor: evidence from brain regional sensitivity to amphetamine. Eur J Neurosci 51(4):1026–1041
Marchese NA, Artur de laVillarmois E, Basmadjian OM, Perez MF, Baiardi G, Bregonzio C (2016) Brain Angiotensin II AT1 receptors are involved in the acute and long-term amphetamine-induced neurocognitive alterations. Psychopharmacology 233(5):795–807
Fanous S, Lacagnina MJ, Nikulina EM, Hammer RP Jr (2011) Sensitized activation of Fos and brain-derived neurotrophic factor in the medial prefrontal cortex and ventral tegmental area accompanies behavioral sensitization to amphetamine. Neuropharmacology 61(4):558–564
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Tan SE (2003) Prenatal amphetamine exposure alters behavioral reactivity to amphetamine in rats. Neurotoxicol Teratol 25(5):579–585
Lyon M, McClure WO (1994) Investigations of fetal development models for prenatal drug exposure and schizophrenia. Prenatal d-amphetamine effects upon early and late juvenile behavior in the rat. Psychopharmacology 116(2):226–236
Nasif FJ, Cuadra GR, Ramirez OA (1999) Permanent alteration of central noradrenergic system by prenatally administered amphetamine. Brain Res Dev Brain Res 112(2):181–188
Uban KA, Comeau WL, Bodnar T, Yu WK, Weinberg J, Galea LA (2015) Amphetamine sensitization and cross-sensitization with acute restraint stress: impact of prenatal alcohol exposure in male and female rats. Psychopharmacology 232(10):1705–1716
Uban KA, Comeau WL, Ellis LA, Galea LA, Weinberg J (2013) Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology 38(10):1953–1966
Segal DM, Moraes CT, Mash DC (1997) Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res 45(2):335–339
Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N (2001) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158(12):2015–2021
Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP (1993) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14(2):169–177
Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS (2012) Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17(9):918–925
Sun H, Calipari ES, Beveridge TJ, Jones SR, Chen R (2015) The brain gene expression profile of dopamine D2/D3 receptors and associated signaling proteins following amphetamine self-administration. Neuroscience 307:253–261
Miyamoto Y, Nagayoshi I, Nishi A, Fukuda T (2019) Three divisions of the mouse caudal striatum differ in the proportions of dopamine D1 and D2 receptor-expressing cells, distribution of dopaminergic axons, and composition of cholinergic and GABAergic interneurons. Brain Struct Funct 224(8):2703–2716
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16(3):223–244
Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M (1983) Long-term sensitization to the excitatory effects of morphine. A motility study in post-dependent rats. Neuropharmacology 22(10):1193–1196
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396(2):157–198
Antelman SM, Eichler AJ, Black CA, Kocan D (1980) Interchangeability of stress and amphetamine in sensitization. Science 207(4428):329–331
Piazza PV, Deminiere JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245(4925):1511–1513
Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46(2):122–126
Robbins SJ, Ehrman RN, Childress AR, O’Brien CP (1999) Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend 53(3):223–230
Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD Sr (2006) Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med 3(3):206–222
van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A (2009) Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol 31(6):706–719
Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C (1997) Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 44(1):35–40
Schindler CW, Bross JG, Thorndike EB (2002) Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol 442(3):231–235
Becker JB (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64(4):803–812
Cailhol S, Mormede P (1999) Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res 842(1):200–205
Carlsson M, Svensson K, Eriksson E, Carlsson A (1985) Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neural Transm 63(3–4):297–313
Gordon JH, Shellenberger K (1974) Regional catecholamine content in the rat brain: sex differences and correlation with motor activity. Neuropharmacology 13(2):129–137
Fleckenstein AE, Haughey HM, Metzger RR, Kokoshka JM, Riddle EL, Hanson JE, Gibb JW, Hanson GR (1999) Differential effects of psychostimulants and related agents on dopaminergic and serotonergic transporter function. Eur J Pharmacol 382(1):45–49
Rothman RB, Baumann MH (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479(1–3):23–40
Acknowledgements
The authors are grateful to Ms. E. G de DiNasso and Mr. J. Rosales for their skillful technical assistance and Dr. Mariella Superina for her skillful assistance in manuscript language revision.
Funding
This work has been supported by grants SeCTy P J/492 and SIIP 06/J520 from the Universidad Nacional de Cuyo, Mendoza, Argentina and PIP 081-2015 from CONICET.
Author information
Authors and Affiliations
Contributions
PGE: conducted real time PCR, collaborated in methodology writing, reviewed the manuscript. SFE: participated in the animal experiment, collaborated in surgical procedure and locomotor activity recording. NFJ: participated in the animal experiment, collaborated in locomotor activity recording. BC: conceptualized and organized the experiments, data curation, writing-reviewing and editing the manuscript. SM: conceptualized and organized the experiments, data curation, writing-reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical Approval
All experimental procedures were approved by the Care and Use of Laboratory Animals Committee (CICUAL) of the Faculty of Medical Sciences, National University of Cuyo, Mendoza, Argentina.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pennacchio, G.E., Santonja, F.E., Neira, F.J. et al. Prenatal Amphetamine-Induced Dopaminergic Alteration in a Gender- and Estrogen-Dependent Manner. Neurochem Res 47, 1317–1328 (2022). https://doi.org/10.1007/s11064-022-03531-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03531-1