Abstract
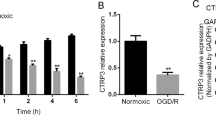
Cerebral ischemia is a major cause of morbidity and permanent disability. To date, no treatments for cerebral ischemia/reperfusion injury can be effectively administered beyond 4–6 h after the ischemic insult. Our study aimed to clarify the significance of Sirt3 during acute cerebral ischemia and explore Sirt3-targeted therapy for ischemic injuries. Upon establishing the oxygen–glucose deprivation/reperfusion (OGD/R) cell model, changes of Sirt3 protein levels and the effects of Sirt3 overexpression on primary hippocampal neurons were detected at indicated time points. Moreover, mitochondrial damage was observed in neurons upon OGD/R injury. The results showed that compared with the normoxia group, Sirt3 protein was significantly decreased in hippocampal neurons exposed to 1 h of OGD followed by 12 h of reperfusion. In addition, the reduction of Sirt3 protein levels contributed to OGD/R-induced neuronal injuries, a higher ratio of neuronal apoptosis, and extensive production of reactive oxygen species (ROS). However, all neuronal injuries were partly rescued by Sirt3 overexpression induced by lentivirus transfection. Mitochondrial morphologies were significantly impaired after OGD/R, but partly salvaged by Sirt3 overexpression. We further explored whether pharmacologically activating Sirt3 is protective for neurons, and found that treatment with honokiol (a Sirt3 agonist) after OGD exposure activated Sirt3 during reperfusion and significantly alleviated OGD/R-induced neuronal injuries. Because mitochondrial functions are essential for neuronal survival, the current results indicate that Sirt3 may be an efficient target to suppress ischemic injuries via maintenance of mitochondrial homeostasis. Our current findings shed light on a novel therapeutic strategy against subacute ischemic injuries.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Wang S, Shi X, Li H, Pang P, Pei L, Shen H, Lu Y (2017) DAPK1 signaling pathways in stroke: from mechanisms to therapies. Mol Neurobiol 54:4716–4722
Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 14:469–477
Graham SH, Chen J (2001) Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 21:99–109
Mayevsky A, Kutai-Asis H, Tolmasov M (2020) Mitochondrial function and brain metabolic score (BMS) in ischemic stroke: evaluation of “neuroprotectants” safety and efficacy. Mitochondrion 50:170–194
Kermer P, Liman J, Weishaupt JH, Bahr M (2004) Neuronal apoptosis in neurodegenerative diseases: from basic research to clinical application. Neurodegener Dis 1:9–19
Mira RG, Cerpa W (2020) Building a bridge between NMDAR-mediated excitotoxicity and mitochondrial dysfunction in chronic and acute diseases. Cell Mol Neurobiol 41:1413–1430
Robertson CL, Soane L, Siegel ZT, Fiskum G (2006) The potential role of mitochondria in pediatric traumatic brain injury. Dev Neurosci 28:432–446
Sims NR, Anderson MF (2002) Mitochondrial contributions to tissue damage in stroke. Neurochem Int 40:511–526
Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M, Xiong L, Zhao G (2011) Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 178:169–180
Zheng JH, Xie L, Li N, Fu ZY, Tan XF, Tao R, Qin T, Chen MH (2019) PD98059 protects the brain against mitochondrial-mediated apoptosis and autophagy in a cardiac arrest rat model. Life Sci 232:116618
Gong L, Tang Y, An R, Lin M, Chen L, Du J (2017) RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis 8:e3080–e3080
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17:2481–2495
Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ (2006) Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol 291:C1082–C1088
Haroon S, Vermulst M (2016) Linking mitochondrial dynamics to mitochondrial protein quality control. Curr Opin Genet Dev 38:68–74
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman S, Verdin E, Schwer B (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27:8807–8814
Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49:186–199
Huang W, Huang Y, Huang RQ, Huang CG, Wang WH, Gu JM, Dong Y (2016) SIRT3 expression decreases with reactive oxygen species generation in rat cortical neurons during early brain injury induced by experimental subarachnoid hemorrhage. Biomed Res Int 2016:8263926
Wang Q, Xu J, Li X, Liu Z, Han Y, Xu X, Li X, Tang Y, Liu Y, Yu T, Li X (2019) Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J Cell Physiol 234:23495–23506
Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM (2014) SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 306:H1602-1609
Yan W, Fan J, Zhang X, Song H, Wan R, Wang W, Yin Y (2021) Decreased neuronal synaptosome associated protein 29 contributes to poststroke cognitive impairment by disrupting presynaptic maintenance. Theranostics 11:4616–4636
Signorile A, Santeramo A, Tamma G, Pellegrino T, D’Oria S, Lattanzio P, De Rasmo D (2017) Mitochondrial cAMP prevents apoptosis modulating Sirt3 protein level and OPA1 processing in cardiac myoblast cells. Biochim Biophys Acta Mol Cell Res 1864:355–366
Yu H, Guan Q, Guo L, Zhang H, Pang X, Cheng Y, Zhang X, Sun Y (2016) Gypenosides alleviate myocardial ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function in rat heart. Cell Stress Chaperones 21:429–437
Zhi W, Li K, Wang H, Lei M, Guo Y (2020) Melatonin elicits protective effects on OGD/Rinsulted H9c2 cells by activating PGC1alpha/Nrf2 signaling. Int J Mol Med 45:1294–1304
Vaillant-Beuchot L, Mary A, Pardossi-Piquard R, Bourgeois A, Lauritzen I, Eysert F, Kinoshita PF, Cazareth J, Badot C, Fragaki K, Bussiere R, Martin C, Mary R, Bauer C, Pagnotta S, Paquis-Flucklinger V, Buee-Scherrer V, Buee L, Lacas-Gervais S, Checler F, Chami M (2021) Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer’s disease models and human brains. Acta Neuropathol 141:39–65
Kim TS, Jin YB, Kim YS, Kim S, Kim JK, Lee HM, Suh HW, Choe JH, Kim YJ, Koo BS, Kim HN, Jung M, Lee SH, Kim DK, Chung C, Son JW, Min JJ, Kim JM, Deng CX, Kim HS, Lee SR, Jo EK (2019) SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy 15:1356–1375
Gu WG, Brannstrom T, Jiang W, Wester P (1999) A photothrombotic ring stroke model in rats with remarkable morphological tissue recovery in the region at risk. Exp Brain Res 125:171–183
Mennel HD, El-Abhar H, Schilling M, Bausch J, Krieglstein J (2000) Morphology of tissue damage caused by permanent occlusion of middle cerebral artery in mice. Exp Toxicol Pathol 52:395–404
Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X (2018) Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis 5:245–255
Xiang RP, Zhou MJ, Cui R, Yu HY, Chen Q, Huang YJ, Li Z, Yu C (2021) Effects of different degrees of carotid artery stenosis on the expression of XIAP and Smac in the ischemic penumbra of rats with cerebral ischemia-reperfusion. J Stroke Cerebrovasc Dis 30:1016
Tehse J, Taghibiglou C (2019) The overlooked aspect of excitotoxicity: Glutamate-independent excitotoxicity in traumatic brain injuries. Eur J Neurosci 49:1157–1170
Zhang K, Tu M, Gao W, Cai X, Song F, Chen Z, Zhang Q, Wang J, Jin C, Shi J, Yang X, Zhu Y, Gu W, Hu B, Zheng Y, Zhang H, Tian M (2019) Hollow prussian blue nanozymes drive neuroprotection against ischemic stroke via attenuating oxidative stress, counteracting inflammation, and suppressing cell apoptosis. Nano Lett 19:2812–2823
Verma R, Ritzel RM, Crapser J, Friedler BD, McCullough LD (2019) Evaluation of the neuroprotective effect of Sirt3 in experimental stroke. Transl Stroke Res 10:57–66
Wang Q, Li L, Li CY, Pei Z, Zhou M, Li N (2015) SIRT3 protects cells from hypoxia via PGC-1alpha- and MnSOD-dependent pathways. Neuroscience 286:109–121
Dai SH, Chen T, Li X, Yue KY, Luo P, Yang LK, Zhu J, Wang YH, Fei Z, Jiang XF (2017) Sirt3 confers protection against neuronal ischemia by inducing autophagy: involvement of the AMPK-mTOR pathway. Free Radic Biol Med 108:345–353
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38:1167–1186
Orellana-Urzua S, Rojas I, Libano L, Rodrigo R (2020) Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des 26:4246–4260
Zhang T, Wu C, Yang X, Liu Y, Yang H, Yuan L, Liu Y, Sun S, Yang J (2019) Pseudoginsenoside-F11 protects against transient cerebral ischemia injury in rats involving repressing calcium overload. Neuroscience 411:86–104
Ryou MG, Mallet RT (2018) An in vitro oxygen-glucose deprivation model for studying ischemia-reperfusion injury of neuronal cells. Methods Mol Biol 1717:229–235
Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, Billings FT, Verdin E, Auwerx J, Harrison DG, Dikalov SI (2020) Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ Res 126(4):439–452
Xin T, Lu C (2020) SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY) 12:16224–16237
Katwal G, Baral D, Fan X, Weiyang H, Zhang X, Ling L, Xiong Y, Ye Q, Wang Y (2018) SIRT3 a major player in attenuation of hepatic ischemia-reperfusion injury by reducing ROS via its downstream mediators: SOD2, CYP-D, and HIF-1alpha. Oxid Med Cell Longev 2018:2976957
Vassilopoulos A, Pennington JD, Andresson T, Rees DM, Bosley AD, Fearnley IM, Ham A, Flynn CR, Hill S, Rose KL, Kim HS, Deng CX, Walker JE, Gius D (2014) SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal 21:551–564
Meng H, Yan WY, Lei YH, Wan Z, Hou YY, Sun LK, Zhou JP (2019) SIRT3 regulation of mitochondrial quality control in neurodegenerative diseases. Front Aging Neurosci 11:313
Torrens-Mas M, Oliver J, Roca P, Sastre-Serra J (2017) SIRT3: oncogene and tumor suppressor in cancer. Cancers 9:90
Cho I, Jeong KH, Zhu J, Choi YH, Cho KH, Heo K, Kim WJ (2019) Sirtuin3 protected against neuronal damage and cycled into nucleus in status epilepticus model. Mol Neurobiol 56:4894–4903
Novgorodov SA, Riley CL, Keffler JA, Yu J, Kindy MS, Macklin WB, Lombard DB, Gudz TI (2016) SIRT3 deacetylates ceramide synthases: implications for mitochondrial dysfunction and brain injury. J Biol Chem 291:1957–1973
Almalki WH, Alzahrani A, Mahmoud El-Daly ME, Fadel Ahmed AHF (2021) The emerging potential of SIRT-3 in oxidative stress-inflammatory axis associated increased neuroinflammatory component for metabolically impaired neural cell. Chem Biol Interact 333:1028
Bai X, Yao L, Ma X, Xu X (2018) Small molecules as SIRT modulators. Mini Rev Med Chem 18:1151–1157
Funding
The research leading to these results received funding from the National Natural Science Foundation of China under Grant Agreement No 31771292, 31571162 and from Natural Science Foundation of Beijing Municipality under Grant Agreement No 7202006.
Author information
Authors and Affiliations
Contributions
Conceptualization: RW, JF, YY; Data curation: HS, JF; Formal analysis: WS; Funding acquisition: YY; Investigation: RW, JF; Project administration: YY; Resources: YY; Supervision: WS; Validation: HS; Visualization: HS, JF; Writing—original draft: RW, WS; Writing—review & editing: RW, YY. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Research Involving Humans and Animal Participants
All procedures involving care and operations of animals were approved by the Ethics Committee of Institutional Animal Care and Use Committee (IACUC), and followed the Code of Practice for the Housing and Care of Animals Used in Scientific Procedures (Protocol Code AEEI-2018–024).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, R., Fan, J., Song, H. et al. Oxygen–Glucose Deprivation/Reperfusion-Induced Sirt3 Reduction Facilitated Neuronal Injuries in an Apoptosis-Dependent Manner During Prolonged Reperfusion. Neurochem Res 47, 1012–1024 (2022). https://doi.org/10.1007/s11064-021-03502-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03502-y