Abstract
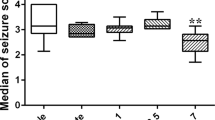
Alpha2-adrenoreceptor (α2-AR) is a noradrenergic receptor that is frequently studied for modulation of seizure activity. However, the precise role of this receptor agonists in regulating seizure activity is still unclear. Our aim in this study was to investigate the effects of α2-AR agonist dexmedetomidine (DEX) and atipamezole (α2-AR antagonist, ATI) on seziures in rats. In the study, 32 adult male Wistar Albino rats (weighing 220–260 g) were used. To induce seizures in rats, pentylenetetrazole (PTZ, 35 mg/kg) was injected intraperitoneally (i.p.) and seizure stages were determined according to the Racine scale. After induction of seizures, DEX (0.1 mg/kg, i.p.) and ATI (1 mg/kg, i.p.) were administered to rats and their effects determined on seizures. GABA levels of the brain hippocampal tissue sample were measured using an ELISA kit and c-Fos positive cells of the dentate gyrus and hippocampal regions were quantitatively analyzed with Image J software. The results showed that DEX decreased the seizure stages according to the Racine scale, significantly prolonged the onset time of first myoclonic jerk (FMJ) and reduced the number of spikes and percentage seizure duration (p < 0.05). In contrast, ATI increased the seizure stage, the number of spikes and percentage seizure duration. The hippocampal GABA level was significantly decreased in rats with only PTZ injection (p < 0.05). In addition, DEX reduced the number of c-Fos positive cells in dentate gyrus and the hippocampal CA1 and CA3 regions. In conclusion, our findings showed that α2-AR agonist DEX had a reducing activity on PTZ-induced seizure, while α2-AR antagonist ATI facilitated seizure formation.







Similar content being viewed by others
Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Weltha L, Reemmer J, Boison D (2019) The role of adenosine in epilepsy. Brain Res Bull 151:46–54
Giorgi FS, Pizzanelli C, Biagioni F, Murri L, Fornai F (2004) The role of norepinephrine in epilepsy: from the bench to the bedside. Neurosci Biobehav Rev 28:507–524
Moezi L, Mansoori E, Niknahad H, Shafaroodi H (2014) The role of alpha-2 adrenoceptors in the anticonvulsant effects of adenosine on pentylenetetrazole-induced seizure threshold in mice. Pharmacol Biochem Behav 126:36–42
Meldrum BS, Akbar MT, Chapman AG (1999) Glutamate receptors and transporters in genetic and acquired models of epilepsy. Epilepsy Res 36:189–204
Gunes H, Ozdemir E, Arslan G (2019) Coenzyme Q10 increases absence seizures in WAG/Rij rats: The role of the nitric oxide pathway. Epilepsy Res 154:69–73
Taskıran AS, Ozdemir E, Gumus E, Ergul M (2020) The effects of salmon calcitonin on epileptic seizures, epileptogenesis, and postseizure hippocampal neuronal damage in pentylenetetrazole-induced epilepsy model in rats. Epilepsy Behav 113:107501
Loscher W, Kohling R (2010) Functional, metabolic, and synaptic changes after seizures as potential targets for antiepileptic therapy. Epilepsy Behav 19:105–113
Strac DS, Pivac N, Smolders IJ, Fogel WA, Deurwaerdere PD, Giovanni GD (2016) Monoaminergic mechanisms in epilepsy may offer innovative therapeutic opportunity for monoaminergic multi-target drugs. Front Neurosci 10:492
Szot P, Lester M, Laughlin ML, Palmiter RD, Liles LC, Weinshenker D (2004) The anticonvulsant and proconvulsant effects of α2-adrenoreceptor agonists are mediated by distinct populations of α2a-adrenoreceptors. Neuroscience 126:795–803
Van Gaalen M, Kawahara H, Kawahara Y, Westerink BHC (1997) The locus coeruleus noradrenergic system in the rat brain studied by dual-probe microdialysis. Brain Res 763:56–62
Kawahara Y, Kawahara H, Westernik BHC (1999) Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studies by dual-probe microdialysis. Brain Res 823:42–48
Bucheler MM, Hadamek K, Hein L (2002) Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience 109:819–826
Amabeoku G, Chikuni O, Bwakura E (1994) Gamma aminobutyric acid mediation of the anticonvulsant effect of clonidine on pentylenetetrazol-induced seizures in mice. Pharmacol Res 29:273–280
Fletcher A, Forster EA (1988) A proconvulsant action of selective alpha 2-adrenoceptor antagonists. Eur J Pharmacol 151:27–34
Loscher W, Czuczwar SJ (1987) Comparison of drugs with different selectivity for central alpha 1-and alpha 2-adrenoceptors in animal models of epilepsy. Epilepsy Res 1:165–172
Chermat R, Doare L, Lachapelle F, Simon P (1981) Effects of drugs affecting the noradrenergic system on convulsions in the quaking mouse. Naunyn Schmiedebergs Arch Pharmacol 318:94–99
Tacke U, Kolonen S (1984) The effect of clonidine and yohimbine on audiogenic seizures (AGS) in rats. Pharmacol Res Commun 16:1019–1030
Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF (1991) Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology 75:328–332
Whittington RA, Virag L, Vulliemoz Y, Cooper TB, Morishima HO (2002) Dexmedetomidine increases the cocaine seizure threshold in rats. Anesthesiology 97:693–700
Zhai M-Z, Wu H-H, Yin J-B, Cui Y-Y, Mei X-P, Zhang H et al (2016) Dexmedetomidine dose-dependently attenuates ropivacaine-induced seizures and negative emotions via inhibiting phosphorylation of amygdala extracellular signal-regulated kinase in mice. Mol Neurobiol 53:2636–2646
Wang L, Tang S, Wang Z, Chen H, Rajcha SS, Qian J (2019) The administration of dexmedetomidine changes microRNA expression profiling of rat hearts. Biomed Pharmacother 120:109463
Nissinen J, Andrade P, Natunen T, Hiltunen M, Malm T, Kanninen K et al (2017) Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res 136:18–34
Mirsky MAZ, Rossell LA, McPherson RW, Traystman RY (1994) Dexmedetomidine decreases seizure threshold in a rat model of experimental generalized epilepsy. Anesthesiology 81:1422–1428
Srivastava AK, Alex AB, Wilcox KS, White HS (2013) Rapid loss of efficacy to the antiseizure drugs lamotrigine and carbamazepine: a novel experimental model of pharmacoresistant epilepsy. Epilepsia 54:1186–1194
Treiman DM, Walton NY, Kendrick C (1990) A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res 5:49–60
Davoudi M, Shojaei A, Palizvan MR, Javan M, Mirnajafi-Zadeh J (2013) Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res 106:54–63
Paxinos G, Watson C (1988) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Weiss R, Tomasula JJ, Sotolongo JR (1990) The effect of an alpha-2 agonist on bladder function and cord histology after spinal cord injury. J Urol 144:1527–1530
Fornai F, Ruffoli R, Giorgi FS, Paparelli A (2011) The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur J Neurosci 33:2169–2178
Corda MG, Orlandi M, Lecca D, Carboni G et al (1991) Pentylenetetrazol-induced kindling in rats: effect of GABA function inhibitors. Pharmacol Biochem Behav 40:329–333
Kulkarni SK (1981) Actions of clonidine on convulsions and behaviour. Arch Int Pharmacodyn Ther 252:124–132
Homayoun H, Khavandgar S, Dehpour AR (2002) The role of alpha2-adrenoceptors in the modulatory effects of morphine on seizure susceptibility in mice. Epilepsia 43:797–804
Lazarova M, Bendotti C, Samanin R (1984) Evidence that the dorsal raphe area is involved in the effect of clonidine against pentylenetetrazole-induced seizures in rats. Naunyn Schmiedebergs Arch Pharmacol 325:12–16
Halonen T, Kotti T, Tuunanen J, Toppinen A, Miettinen R, Riekkinen PJ (1995) α2-Adrenoceptor agonist, dexmedetomidine, protects against kainic acid-induced convulsions and neuronal damage. Brain Res 693:217–224
Savola JM, Virtanen R (1991) Central alpha2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol 195:193–199
Maier C, Steinberg GK, Sun GH, Zhi GT, Maze M (1993) Neuroprotection by the a2-adrenoreceptor agonist dexmedetomidine in a focal model of cerebral ischemia. Anesthesiology 79:306–312
Freund TF, Ylinen A, Miettinen R, Pitkinen A et al (1991) Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull 28:27–38
Rowley HL, Martin KF, Marsden CA (1995) Decreased GABA release following tonic-clonic seizures is associated withan increase in extracellular glutamate in rat hippocampus in vivo. Neuroscience 68:415–422
Valtonen P, Haapalinna A, Riekkinen P, Haionen T (1995) Effect of alfa-2-adrenergic drugs dexmedetomidine and atipamezole on extracellular amino acid levels in vivo. Eur J Pharmacol 285:239–246
Malhi SM, Jawed H, Hanif F, Ashraf M, Zubair F et al (2014) Modulation of c-Fos and BDNF protein expression in pentylenetetrazol kindled mice following the treatment with novel antiepileptic compound HHL-6. Biomed Res Int 2014:876712
Erdtmann-Vourliotis M, Riechter U, Mayer P, Grecksch G, Höllt V (1998) Pentylenetetrazol (PTZ)-induced c-fos expression in the hippocampus of kindled rats is suppressed by concomitant treatment with naloxone. Brain Res 792:299–308
Roe DL, Bardgett ME, Csernansky CA, Csernansky JG (1998) Induction of Fos protein by antipsychotic drugs in rat brain following kainic acid-induced limbic-cortical neuronal loss. Psychopharmacology 138(2):151–158
Jurgens CWD, Hammad HM, Lichter JA, Boese SJ, Nelson BW, Goldenstein BL et al (2007) α2A-adrenergic receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region. Mol Pharmacol 71:1572–1581
Motte JE, da Silva Fernandes MJ, Marescaux C, Nehlig A (1997) Effects of pentylenetetrazol-induced status epilepticus on c-Fos and HSP72 immunoreactivity in the immature rat brain. Mol Brain Res 50:79–84
Funding
This study was supported by Cumhuriyet University Scientific Research Project, Sivas, Turkey (CUBAP, Grant Number: T-718).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cetindag Ciltas, A., Ozdemir, E., Gumus, E. et al. The Anticonvulsant Effects of Alpha-2 Adrenoceptor Agonist Dexmedetomidine on Pentylenetetrazole-Induced Seizures in Rats. Neurochem Res 47, 305–314 (2022). https://doi.org/10.1007/s11064-021-03445-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03445-4