Abstract
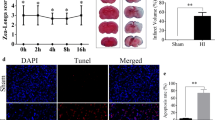
Mild intrauterine hypoperfusion (MIUH) can induce placental dysfunction and lead to long-term changes during the process of brain development. A better understanding of the mechanism of MIUH will help in the development of new neuroprotective strategies for the placental chamber. To better understand the mechanism of the effect of MIUH on the neural development of offspring, we constructed a model of MIUH in pregnant rats. The proliferation, apoptosis, and autophagy of hippocampal neurons in fetal rats were studied via flow cytometry, immunofluorescence staining, JC-1 staining, western blotting, and real-time polymerase chain reaction at different time points (6, 24, 48, and 72 h). The results showed that MIUH significantly inhibited the proliferation of hippocampal neurons and promoted their apoptosis and autophagy. Simultaneously, MIUH could promote PTEN expression and affect the PTEN signaling pathway. bpV, an inhibitor of PTEN, could restore the inhibition of hippocampal nerve cell growth caused by MIUH. MIUH may inhibit neuronal proliferation and promote neuronal apoptosis and autophagy by regulating the PTEN signaling pathway.




Similar content being viewed by others
Data Availability
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Hampl V, Jakoubek V (2009) Regulation of fetoplacental vascular bed by hypoxia. Physiol Res 58(Suppl 2):S87-93
Tsuji M, Coq JO, Ogawa Y, Yamamoto Y, Ohshima M (2018) A rat model of mild intrauterine hypoperfusion with microcoil stenosis. J Vis Exp. https://doi.org/10.3791/56723
Ohshima M, Coq JO, Otani K, Hattori Y, Ogawa Y, Sato Y, Harada-Shiba M, Ihara M, Tsuji M (2016) Mild intrauterine hypoperfusion reproduces neurodevelopmental disorders observed in prematurity. Sci Rep 6:39377
Coq JO, Delcour M, Ogawa Y, Peyronnet J, Castets F, Turle-Lorenzo N, Montel V, Bodineau L, Cardot P, Brocard C, Liabeuf S, Bastide B, Canu MH et al (2018) Mild intrauterine hypoperfusion leads to lumbar and cortical hyperexcitability, spasticity, and muscle dysfunctions in rats: implications for prematurity. Front Neurol 9:423
Ciurea EL, Berceanu C, Voicu NL, Pirnoiu D, Berceanu S, Stepan AE (2018) Morphological survey of placenta in trombophilia related hypoperfusion of maternal-fetal blood flow. Curr Health Sci J 44:85–91
Catteau J, Gernet JI, Marret S, Legros H, Gressens P, Leroux P, Laudenbach V (2011) Effects of antenatal uteroplacental hypoperfusion on neonatal microvascularisation and excitotoxin sensitivity in mice. Pediatr Res 70:229–235
Tolcos M, Markwick R, O’Dowd R, Martin V, Turnley A, Rees S (2015) Intrauterine growth restriction: effects on neural precursor cell proliferation and angiogenesis in the foetal subventricular zone. Dev Neurosci 37:453–463
Gilles F, Gressens P, Dammann O, Leviton A (2018) Hypoxia-ischemia is not an antecedent of most preterm brain damage: the illusion of validity. Dev Med Child Neurol 60:120–125
Skelton PD, Stan RV, Luikart BW (2020) The role of PTEN in neurodevelopment. Mol Neuropsychiatry 5:60–71
Yin S-W, Wang Y, Meng Y-L, Liu C-X (2020) Effects of mild intrauterine hypoperfusion in the second trimester on memory and learning function in rat offspring. Neural Regen Res 15:2082–2088. https://doi.org/10.4103/1673-5374.282268
Wang Y, Yin SW, Zhang N, Zhao P (2018) High-concentration sevoflurane exposure in mid-gestation induces apoptosis of neural stem cells in rat offspring. Neural Regen Res 13:1575–1584
Wang Y, Yin S, Xue H, Yang Y, Zhang N, Zhao P (2018) Mid-gestational sevoflurane exposure inhibits fetal neural stem cell proliferation and impairs postnatal learning and memory function in a dose-dependent manner. Dev Biol 435:185–197
Wang Y, Li XW, Liu J, Fu W (2018) Antenatal taurine supplementation in fetal rats with growth restriction improves neural stem cell proliferation by inhibiting the activities of Rho family factors. J Matern Fetal Neonatal Med 31:1454–1461
Yan H, Zhang X, Luo S, Liu H, Wang X, Gao Y, Wilson JX, Huang G (2013) Effects of homocysteine on ERK signaling and cell proliferation in fetal neural stem cells in vitro. Cell Biochem Biophys 66:131–137
Hong CJ, Park H, Yu SW (2016) Autophagy for the quality control of adult hippocampal neural stem cells. Brain Res 1649:166–172
Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K (2010) Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 30:1017–1030
Wang S, Xu X, Hu Y, Lei T, Liu T (2019) Sotetsuflavone induces autophagy in non-small cell lung cancer through blocking PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Front Pharmacol 10:1460
Han J, Huang C, Jiang J, Jiang D (2019) Activation of autophagy during farnesyl pyrophosphate synthase inhibition is mediated through PI3K/AKT/mTOR signaling. J Int Med Res. https://doi.org/10.1177/0300060519875371
Yunan Gao Hu, Qiao ZL, Hou Y (2019) miR29 promotes the proliferation of cultured rat neural stem/progenitor cells via the PTEN/AKT signaling pathway. Mol Med Rep 20:2111–2118
Acknowledgements
This work was supported by National Key R&D Program of China (2018YFC1002902) to CL.
Funding
This work was supported by National Key R&D Program of China (2018YFC1002902) to CL.
Author information
Authors and Affiliations
Contributions
SY, YW, and YM designed research; SY, YW, and YM performed research; SY, YW analyzed data; SY, CL wrote the paper, YW obtained funding, administrative, technical or material support, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
The procedures followed were in accordance with Animal Ethics Committee of Shengjing Hospital of China (NO. 2018PS07K). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Consent for Publication
All authors agree to publishing this article in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, S., Meng, Y., Liu, C. et al. MIUH Inhibits the Hippocampal Neuron Growth in Fetal Rat by Affecting the PTEN Pathway. Neurochem Res 46, 2046–2055 (2021). https://doi.org/10.1007/s11064-021-03342-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03342-w