Abstract
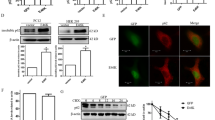
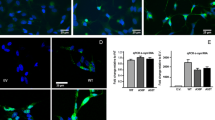
Although the etiology of Parkinson's disease (PD) is multifactorial, it has been linked to abnormal accumulation of α-synuclein (α-syn) in dopaminergic neurons, which could lead to dysfunctions on intracellular organelles, with potential neurodegeneration. Patients with familial early-onset PD frequently present mutation in the α-syn gene (SNCA), which encodes mutant α-syn forms, such as A30P and A53T, which potentially regulate Ca2+ unbalance. Here we investigated the effects of overexpression of wild-type α-syn (WT) and the mutant forms A30P and A53T, on modulation of lysosomal Ca2+ stores and further autophagy activation. We found that in α-syn-overexpressing cells, there was a decrease in Ca2+ released from endoplasmic reticulum (ER) which is related to the increase in lysosomal Ca2+ release, coupled to lysosomal pH alkalization. Interestingly, α-syn-overexpressing cells showed lower LAMP1 levels, and a disruption of lysosomal morphology and distribution, affecting autophagy. Interestingly, all these effects were more evident with A53T mutant isoform when compared to A30P and WT α-syn types, indicating that the pathogenic phenotype for PD is potentially related to impairment of α-syn degradation. Taken together, these events directly impact PD-related dysfunctions, being considered possible molecular targets for neuroprotection.





Similar content being viewed by others
Abbreviations
- α-syn:
-
α-synuclein
- Baf A1:
-
Bafilomycin-A1
- CICR:
-
Calcium-induced calcium release
- ER:
-
Endoplasmic reticulum
- GPN:
-
Glycyl-l-phenylalanine 2-naphthylamide
- IP3R:
-
Inositol 1,4,5-trisphosphate receptor
- LAMP1:
-
Lysosomal membrane-associated protein 1
- MCSs:
-
Membrane contact sites
- NAADP:
-
Nicotinic acid adenine dinucleotide phosphate
- PD:
-
Parkinson’s disease
- RyR:
-
Ryanodine receptor
- SERCA:
-
Sarco/endoplasmic Ca2+-ATPase
- SH-SY5Y:
-
Human neuroblastoma cell line
- SNpc:
-
Substantia nigra pars compacta
- STV:
-
Starvation
- TPCs:
-
Two-pore channels
References
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29:1583–1590. https://doi.org/10.1002/mds.25945
Bernheimer H, Birkmayer W, Hornykiewicz O et al (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455. https://doi.org/10.1016/0022-510x(73)90175-5
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Di Monte DA (2003) The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol 2:531–538. https://doi.org/10.1016/s1474-4422(03)00501-5
Gasser T (2009) Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med 11:e22. https://doi.org/10.1017/S1462399409001148
Uversky VN, Li J, Fink AL (2001) Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett 500:105–108. https://doi.org/10.1016/s0014-5793(01)02597-2
Braak H, Del Tredici K, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Halliday GM, McCann H (2010) The progression of pathology in Parkinson’s disease. Ann N Y Acad Sci 1184:188–195. https://doi.org/10.1111/j.1749-6632.2009.05118.x
Krüger R, Kuhn W, Müller T et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108. https://doi.org/10.1038/ng0298-106
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA (2006) Calcium in cell injury and death. Annu Rev Pathol 1:405–434. https://doi.org/10.1146/annurev.pathol.1.110304.100218
Erustes AG, Stefani FY, Terashima JY et al (2018) Overexpression of α-synuclein in an astrocyte cell line promotes autophagy inhibition and apoptosis. J Neurosci Res 96:160–171. https://doi.org/10.1002/jnr.24092
Fujikake N, Shin M, Shimizu S (2018) Association between autophagy and neurodegenerative diseases. Front Neurosci. https://doi.org/10.3389/fnins.2018.00255
Nicotera P, Orrenius S (1998) The role of calcium in apoptosis. Cell Calcium 23:173–180
Scannevin RH (2018) Therapeutic strategies for targeting neurodegenerative protein misfolding disorders. Curr Opin Chem Biol 44:66–74
Smaili S, Hirata H, Ureshino R et al (2009) Calcium and cell death signaling in neurodegeneration and aging. An Acad Bras Cienc 81:467–475
Gordon PB, Holen I, Fosse M et al (1993) Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem 268:26107–26112
Levine TP, Patel S (2016) Signalling at membrane contact sites: two membranes come together to handle second messengers. Curr Opin Cell Biol 39:77–83. https://doi.org/10.1016/j.ceb.2016.02.011
Prinz WA, Toulmay A, Balla T (2020) The functional universe of membrane contact sites. Nat Rev Mol Cell Biol 21:7–24
Galione A (2006) NAADP, a new intracellular messenger that mobilizes Ca2+ from acidic stores. Biochem Soc Trans 34:922–926. https://doi.org/10.1042/BST0340922
Patel S, Docampo R (2010) Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol 20:277–286. https://doi.org/10.1016/j.tcb.2010.02.003
Cuervo AM, Stafanis L, Fredenburg R et al (2004) Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305:1292–1295. https://doi.org/10.1126/science.1101738
Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z et al (2011) Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci 31:14508–14520. https://doi.org/10.1523/JNEUROSCI.1560-11.2011
Lawrence RE, Zoncu R (2019) The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol 21:133–142
Perera RM, Zoncu R (2016) The lysosome as a regulatory hub. Annu Rev Cell Dev Biol 32:223–253. https://doi.org/10.1146/annurev-cellbio-111315-125125
Shen HM, Mizushima N (2014) At the end of the autophagic road: an emerging understanding of lysosomal functions in autophagy. Trends Biochem Sci 39:61–71
Brailoiu E, Hooper R, Cai X et al (2010) An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem 285:2897–2901. https://doi.org/10.1074/jbc.C109.081943
Brailoiu E, Churamani D, Cai X et al (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol 186:201–209. https://doi.org/10.1083/jcb.200904073
Calcraft PJ, Ruas M, Pan Z et al (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459:596–600. https://doi.org/10.1038/nature08030
Pereira GJS, Tressoldi N, Hirata H et al (2013) Autophagy as a neuroprotective mechanism against 3-nitropropionic acid-induced murine astrocyte cell death. Neurochem Res 38:2418–2426. https://doi.org/10.1007/s11064-013-1154-5
Pereira GJS, Hirata H, Fimia GM et al (2011) Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem 286:27875–27881. https://doi.org/10.1074/jbc.C110.216580
Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T et al (2006) Plasma membrane ion permeability induced by mutant α-synuclein contributes to the degeneration of neural cells. J Neurochem 97:1071–1077. https://doi.org/10.1111/j.1471-4159.2006.03803.x
Morgan AJ, Davis LC, Galione A (2015) Imaging approaches to measuring lysosomal calcium. Methods Cell Biol 126:159–195. https://doi.org/10.1016/bs.mcb.2014.10.031
Pereira GJS, Hirata H, do Carmo LG et al (2014) NAADP-sensitive two-pore channels are present and functional in gastric smooth muscle cells. Cell Calcium 56:51–58. https://doi.org/10.1016/j.ceca.2014.04.005
Jencks WP (1992) On the mechanism of ATP-driven Ca2+ transport by the calcium ATPase of sarcoplasmic reticulum. Ann N Y Acad Sci 671:47–49
Telmer CA, Verma R, Teng H et al (2015) Rapid, specific, no-wash, far-red fluorogen activation in subcellular compartments by targeted fluorogen activating proteins. ACS Chem Biol 10:1239–1246. https://doi.org/10.1021/cb500957k
Adler J, Parmryd I (2010) Quantifying colocalization by correlation: the pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A 77:733–742. https://doi.org/10.1002/cyto.a.20896
Kilpatrick BS, Eden ER, Hockey LN et al (2015) Methods for monitoring lysosomal morphology. Methods Cell Biol 126:1–19. https://doi.org/10.1016/bs.mcb.2014.10.018
Lin HJ, Herman P, Kang JS, Lakowicz JR (2001) Fluorescence lifetime characterization of novel low-pH probes. Anal Biochem 294:118–125. https://doi.org/10.1006/abio.2001.5155
Arotcarena M-L, Teil M, Dehay B (2019) Autophagy in synucleinopathy: the overwhelmed and defective machinery. Cells 8:565. https://doi.org/10.3390/cells8060565
Mazzulli JR, Zunke F, Isacson O et al (2016) α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci USA 113:1931–1936. https://doi.org/10.1073/pnas.1520335113
Ureshino E et al (2019) The interplay between Ca2+ signaling pathways and neurodegeneration. Int J Mol Sci 20:6004. https://doi.org/10.3390/ijms20236004
Lee HC (2003) Calcium signaling: NAADP ascends as a new messenger. Curr Biol 13:R186–R188. https://doi.org/10.1016/s0960-9822(03)00120-9
Berg TO, Strømhaug E, Løvdal T et al (1994) Use of glycyl-L-phenylalanine 2-naphthylamide, a lysosome-disrupting cathepsin C substrate, to distinguish between lysosomes and prelysosomal endocytic vacuoles. Biochem J 300:229–236
Betzer C, Jensen PH (2018) Reduced cytosolic calcium as an early decisive cellular state in Parkinson’s disease and synucleinopathies. Front Neurosci 12:819. https://doi.org/10.3389/fnins.2018.00819
Rivero-Ríos P, Gómez-Suaga P, Fdez E, Hilfiker S (2014) Upstream deregulation of calcium signaling in Parkinson’s disease. Front Mol Neurosci 7:53. https://doi.org/10.3389/fnmol.2014.00053
Volles MJ, Lansbury PT (2002) Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41:4595–4602
Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C (1988) Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107:2587–2600. https://doi.org/10.1083/jcb.107.6.2587
Patel S, Brailoiu E (2012) Triggering of Ca2+ signals by NAADP-gated two-pore channels: a role for membrane contact sites? Biochem Soc Trans 40:153–157. https://doi.org/10.1042/BST20110693
Chu Y, Dodiya H, Aebischer P et al (2009) Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis 35:385–398. https://doi.org/10.1016/j.nbd.2009.05.023
Dehay B, Bové J, Rodríguez-Muela N et al (2010) Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 30:12535–12544. https://doi.org/10.1523/JNEUROSCI.1920-10.2010
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4:552–565. https://doi.org/10.1038/nrm1150
Ishida Y, Nayak S, Mindell JA, Grabe M (2013) A model of lysosomal pH regulation. J Gen Physiol 141:705–720. https://doi.org/10.1085/jgp.201210930
Zhao J, Benlekbir S, Rubinstein JL (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521:241–245. https://doi.org/10.1038/nature14365
Fukuda T, Ewan L, Bauer M et al (2006) Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol 59:700–708. https://doi.org/10.1002/ana.20807
Henry AG, Aghamohammadzadeh S, Samaroo H et al (2015) Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum Mol Genet 24:6013–6028. https://doi.org/10.1093/hmg/ddv314
Dehay B, Martinez-Vicente M, Ramirez A et al (2012) Lysosomal dysfunction in Parkinson disease. Autophagy 8:1389–1391. https://doi.org/10.4161/auto.21011
Coffey EE, Beckel JM, Laties AM, Mitchell CH (2014) Lysosomal alkalization and dysfunction in human fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience 263:111–124. https://doi.org/10.1016/j.neuroscience.2014.01.001
Golabek AA, Kida E, Walus M et al (2000) CLN3 protein regulates lysosomal pH and alters intracellular processing of Alzheimer’s amyloid-beta protein precursor and cathepsin D in human cells. Mol Genet Metab 70:203–213. https://doi.org/10.1006/mgme.2000.3006
Lee JH, Yu WH, Kumar A et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141:1146–1158. https://doi.org/10.1016/j.cell.2010.05.008
Kinouchi K, Ichihara A, Itoh H (2011) Functional characterization of (pro)renin receptor in association with V-ATPase. Front Biosci 16:3216–3223
Stefanis L, Larsen KE, Rideout HJ et al (2001) Expression of A53T mutant but not wild-type α-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci 21:9549–9560. https://doi.org/10.1523/JNEUROSCI.21-24-09549.2001
Ravikumar B, Vacher C, Berger Z et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. https://doi.org/10.1038/ng1362
Schaeffer V, Lavenir I, Ozcelik S et al (2012) Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 135:2169–2177. https://doi.org/10.1093/brain/aws143
Webb JL, Ravikumar B, Atkins J et al (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013. https://doi.org/10.1074/jbc.M300227200
Mustaly-Kalimi S, Littlefield AM, Stutzmann GE (2018) Calcium signaling deficits in glia and autophagic pathways contributing to neurodegenerative disease. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7266
Lei Z, Cao G, Wei G (2019) A30P mutant α-synuclein impairs autophagic flux by inactivating JNK signaling to enhance ZKSCAN3 activity in midbrain dopaminergic neurons. Cell Death Dis 10:1–14. https://doi.org/10.1038/s41419-019-1364-0
Acknowledgements
We thank Marina Yukari Kubota, Cícero Ramos dos Santos, Maria de Lourdes Santos, Elizabeth Kanashiro for technical assistance, and Grant Churchill for providing NAADP-AM.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP: 2013/20073-2; 2019/02821-8 (SSS); 2017/10863-7 (GJSP); 2016/20796-2 (RPU). Conselho Nacional de Desenvolvimento Científico e Tecnológico: Universal 421603/2018-6 (GJSP); PVE 401236/2014-5 (SSS); PVE 401141/2014-4 (CB). The Master Fellowship (ACN) was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES). Confocal microscope Zeiss LSM 780, facility from the Instituto de Farmacologia e Biologia Molecular (INFAR) was supported by Financiadora de Estudos e Projetos (FINEP) and FAPESP.
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: GJSP, SSS. Acquisition of data: ACN, AGE. Analysis and interpretation of data: ACN, AGE, GJSP, PR, CB. Drafting of the manuscript: ACN, AGE, PR, CB, RPU. Critical revision of the manuscript for important intellectual content: GJSP, SSS. Statistical analysis: ACN. Study supervision: GJSP, SSS.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nascimento, A.C., Erustes, A.G., Reckziegel, P. et al. α-Synuclein Overexpression Induces Lysosomal Dysfunction and Autophagy Impairment in Human Neuroblastoma SH-SY5Y. Neurochem Res 45, 2749–2761 (2020). https://doi.org/10.1007/s11064-020-03126-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03126-8