Abstract
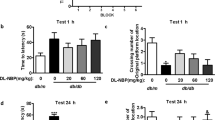
Diabetes mellitus is a prevalent metabolic disorder associated with multiple complications including neuropathy, memory loss and cognitive decline. Despite a long history of studies on diabetic complications, there are no effective therapeutic strategies for neuroprotection in diabetes. Hyperglycemia-induced imbalance in programmed cell death could initiate a decline in neural tissue cells viability. Various nanomaterials can induce either cell death or cell survival dependent on the type and surface features. Pristine C60 fullerene is a nontoxic nanomaterial, which exhibits antioxidant and cytoprotective properties. However, the precise molecular mechanism with which the C60 nanoparticle exerts cytoprotective effect in diabetic subjects has not yet been fully addressed. Thus, this study aimed to determine whether C60 fullerene prevents oxidative stress impairment and to explore the effects of C60 fullerene on apoptosis and autophagy in diabetes mellitus to clarify its potential mechanisms. These effects have been examined for olive oil extracted C60 fullerene on the hippocampus of STZ diabetic rats. Up-regulation of Caspase-3, Beclin-1 and oxidative stress indexes and down-regulation of Bcl-2 were observed in the brain of STZ-diabetic rats. The exposure to C60 fullerene for a period of 12 weeks ameliorate redox imbalance, hyperglycemia-induced disturbances in apoptosis and autophagy flux via modulation of Caspase-3, Bcl-2, Beclin-1 and LC3I/II contents. Furthermore, C60 fullerene ameliorated the LC3I/II ratio and prevented extremely increased autophagy flux. Contrarily, pristine C60 fullerene had no modulatory effect on all studied apoptotic and autophagy markers in non-diabetic groups. Therefore, oil extracted C60 fullerene exhibits cytoprotective effect in hyperglycemia-stressed hippocampal cells. The presented results confirm that pristine C60 fullerene nanoparticles can protect hippocampal cells against hyperglycemic stress via anti-oxidant, anti-apoptotic effects and amelioration of autophagy flux. Moreover, C60 fullerene regulates a balance of autophagy via BCL-2/Beclin-1 reciprocal expression that could prevent functional disturbances in hippocampus.



Similar content being viewed by others
References
Rababa'h AM, Mardini AN, Alzoubi KH, Ababneh MA, Athamneh RY (2019) The effect of cilostazol on hippocampal memory and oxidative stress biomarkers in rat model of diabetes mellitus. Brain Res 1715:182–187
Ye S, Chen M, Jiang Y, Chen M, Zhou T, Wang Y, Hou Z, Ren L (2014) Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defence system. Int J Nanomedicine 9:2073–2087
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC (2018) Neuronal cell death. Physiol Rev 98(2):813–880
Farbood Y, Ghaderi S, Rashno M, Khoshnam SE, Khorsandi L, Sarkaki A, Rashno M (2019) Sesamin: a promising protective agent against diabetes-associated cognitive decline in rats. Life Sci 230:169–177
Lee SC, Pervaiz S (2007) Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol 39:497–504
Soleymaninejad M, Joursaraei SG, Feizi F, Jafari Anarkooli I (2017) The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J Diabetes Res 2017:4650939
Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, Boada J, Prat J, Portero-Otin M, Pamplona R (2012) Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res 2012:696215
Staricha K, Meyers N, Garvin J, Liu Q, Rarick K, Harder D, Cohen S (2020) Effect of high glucose condition on glucose metabolism in primary astrocytes. Brain Res 1732:146702
Bahniwal M, Little JP, Klegeris A (2017) High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res 14(7):731–741
Takahashi S, Izawa Y, Suzuki N (2012) Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro 4(2):e00078
Zhou W, Yao Y, Li J, Wu D, Zhao M, Yan Z, Pang A, Kong L (2019) TIGAR attenuates high glucose-induced neuronal apoptosis via an autophagy pathway. Front Mol Neurosci 12:193
Agca CA, Tuzcu M, Hayirli A, Sahin K (2014) Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol 71:116–121
Schiavone S, Jaquet V, Trabace L, Krause KH (2013) Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal 18(12):1475–1490
Muriach M, Flores-Bellver M, Romero FJ, Barcia JM (2014) Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev 2014:102158
Li W, Roy Choudhury G, Winters A, Prah J, Lin W, Liu R, Yang SH (2018) Hyperglycemia alters astrocyte metabolism and inhibits astrocyte proliferation. Aging Dis 9(4):674–684
Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA (2018) Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 9(2):119
Vincent AM, McLean LL, Backus C, Feldman EL (2005) Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J 19(6):638–640
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29(5):347–364. https://doi.org/10.1038/s41422-019-0164-5
El-Khattouti A, Selimovic D, Haikel Y, Hassan M (2013) Crosstalk between apoptosis and autophagy: molecular mechanisms and therapeutic strategies in cancer. J Cell Death 6:37–55
Huang CY, Kuo WW, Wang HF, Lin CJ, Lin YM, Chen JL, Kuo CH, Chen PK, Lin JY (2014) GABA tea ameliorates cerebral cortex apoptosis and autophagy in streptozotocin-induced diabetic rats. Journal of Functional Foods 6:534–544
Sowers JR, Zhang Y (2018) Autophagy and cardiometabolic diseases: from molecular mechanisms to translational medicine (Zhang, Y. Section II). Academic Press, New York, pp 83–90
Asadi F, Jamshidi AH, Khodagholi F, Yans A, Azimi L, Faizi M, Vali L, Abdollahi M, Ghahremani MH, Sharifzadeh M (2015) Reversal effects of crocin on amyloid β-induced memory deficit: modification of autophagy or apoptosis markers. Pharmacol Biochem Behav 139(Pt A):47–58
Gonzalez CD, Lee MS, Marchetti P, Pietropaolo M, Towns R, Vaccaro MI, Watada H, Wiley JW (2011) The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 7(1):2–11
Wang F, Xu C, Reece EA, Li X, Wu Y, Harman C, Yu J, Dong D, Wang C, Yang P, Zhong J, Yang P (2017) Protein kinase C-alpha suppresses autophagy and induces neural tube defects via miR-129-2 in diabetic pregnancy. Nat Commun 8:15182
Butler D, Bahr BA (2006) Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxid Redox Signal 8(1–2):185–196
Tezil T, Basaga H (2014) Modulation of cell death in age-related diseases. Curr Pharm Des 20(18):3052–3067
Infante-Garcia C, Garcia-Alloza M (2019) Review of the effect of natural compounds and extracts on neurodegeneration in animal models of diabetes mellitus. Int J Mol Sci 20(10):E2533
Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R (2015) Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov 14(1):45–57
Rambold AS, Lippincott-Schwartz J (2011) Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 10(23):4032–4038
Cordani M, Somoza Á (2019) Targeting autophagy using metallic nanoparticles: a promising strategy for cancer treatment. Cell Mol Life Sci 76(7):1215–1242
Nedzvetsky V, Andrievsky G, Chachibaia T, Tykhomyrov A (2012) Differences in antioxidant/protective efficacy of hydrated C60 fullerene nanostructures in liver and brain of rats with streptozotocin-induced diabetes. Diabetes Metabol. https://doi.org/10.4172/2155-6156.1000215
Rondags A, Yuen WY, Jonkman MF, Horváth B (2017) Fullerene C60 with cytoprotective and cytotoxic potential: prospects as a novel treatment agent in dermatology? Exp Dermatol 26(3):220–224
Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F (2005) [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 5(12):2578–2585
Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, Bonn GK (2007) Medicinal applications of fullerenes. Int J Nanomed 2(4):639–649
Tykhomyrov AA, Nedzvetsky VS, Klochkov VK, Andrievsky GV (2008) Nanostructures of hydrated C60 fullerene (C60HyFn) protect rat brain against alcohol impact and attenuate behavioral impairments of alcoholized animals. Toxicology 246(2–3):158–165
Etem EO, Bal R, Akağaç AE, Kuloglu T, Tuzcu M, Andrievsky GV, Buran I, Nedzvetsky VS, Baydas G (2014) The effects of hydrated C(60) fullerene on gene expression profile of TRPM2 and TRPM7 in hyperhomocysteinemic mice. J Recept Signal Transduct Res 34(4):317–324
Nedzvetsky VS, Sukharenko EV, Baydas G, Andrievsky GV (2019) Water-soluble C60 fullerene ameliorates astroglial reactivity and TNFa production in retina of diabetic rats. Regul Mech Biosyst 10(4):513–519
Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, Gür S, Nedzvetsky VS, Tykhomyrov AA, Andrievsky GV, Baydas G, Naziroglu M (2011) Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats. Toxicology 282(3):69–81
Ogunyinka BI, Oyinloye BE, Osunsanmi FO, Opoku AR, Kappo AP (2016) Modulatory influence of Parkia biglobosa protein isolate on testosterone and biomarkers of oxidative stress in brain and testes of streptozotocin-induced diabetic male rats. Int J Physiol Pathophysiol Pharmacol 8(3):78–86
Tabatabaei SRF, Ghaderi S, Bahrami-Tapehebur M, Farbood Y, Rashno M (2017) Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomed Pharmacother 96:279–290
Baati T, Bourasset F, Gharbi N, Njim L, Abderrabba M, Kerkeni A, Szwarc H, Moussa F (2012) The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene. Biomaterials 33(19):4936–4946
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Nedzvetskii VS, Berezich VA, Oberniak TI, Zhmareva EN (1986) Characteristics of specific intermediate filament proteins in human brain tumors. Biokhimiia 51(11):1843–1850
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Duncan DB (1957) Multiple range test for correlated and heteroscedastic means. Biometrics 13:164–176
Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V (2010) The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol Sci 114(2):162–182
Prischepa IV, Prokushenkova OG, Nedzvetsky VS (2015) Nanoparticles C60 fullerene prevent reactive gliosis in retina of aged rats under hyperglycemia. Regul Mech Biosyst 6(2):113–118
Nedzvetskii VS, Pryshchepa IV, Tykhomyrov AA, Baydas G (2016) Inhibition of reactive gliosis in the retina of rats with streptozotocin-induced diabetes under the action of hydrated C60 fullerene. Neurophysiology 48(2):130–140
Caletti G, Herrmann AP, Pulcinelli RR, Steffens L, Morás AM, Vianna P, Chies JAB, Moura DJ, Barros HMT, Gomez R (2018) Taurine counteracts the neurotoxic effects of streptozotocin-induced diabetes in rats. Amino Acids 50(1):95–104
Liu X, Mo Y, Gong J, Li Z, Peng H, Chen J, Wang Q, Ke Z, Xie J (2016) Puerarin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Metab Brain Dis 31(2):417–423
Rebai R, Jasmin L, Boudah A (2017) The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices. Brain Res Bull 134:142–150
Lutchmansingh FK, Hsu JW, Bennett FI, Badaloo AV, McFarlane-Anderson N, Gordon-Strachan GM, Wright-Pascoe RA, Jahoor F, Boyne MS (2018) Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 13(6):e0198626
Elshater AA, Haridy MAM, Salman MMA, Fayyad AS, Hammad S (2018) Fullerene C60 nanoparticles ameliorated cyclophosphamide-induced acute hepatotoxicity in rats. Biomed Pharmacother 97:53–59
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122(6):927–939
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571–580
Zhou KL, Zhou YF, Wu K, Tian NF, Wu YS, Wang YL, Chen DH, Zhou B, Wang XY, Xu HZ, Zhang XL (2015) Stimulation of autophagy promotes functional recovery in diabetic rats with spinal cord injury. Sci Rep 5:17130
Maurya SK, Tewari M, Sharma B, Shukla HS (2013) Expression of procaspase 3 and activated caspase 3 and its relevance in hormone-responsive gallbladder carcinoma chemotherapy. Korean J Intern Med 28(5):573–578
Rice KM, Manne ND, Gadde MK, Paturi S, Arvapalli R, Blough E (2015) Differential regulation of apoptosis in slow and fast twitch muscles of aged female F344BN rats. Age (Dordr) 37(2):30
Ummanni R, Lehnigk U, Zimmermann U, Woenckhaus C, Walther R, Giebel J (2010) Immunohistochemical expression of caspase-1 and -9, uncleaved caspase-3 and -6, cleaved caspase-3 and -6 as well as Bcl-2 in benign epithelium and cancer of the prostate. Exp Ther Med 1(1):47–52
Hu Z, Guan W, Wang W, Huang L, Xing H, Zhu Z (2007) Protective effect of a novel cystine C(60) derivative on hydrogen peroxide-induced apoptosis in rat pheochromocytoma PC12 cells. Chem Biol Interact 167(2):135–144
Markovic Z, Trajkovic V (2008) Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 29(26):3561–3573
Althunibat OY, Al Hroob AM, Abukhalil MH, Germoush MO, Bin-Jumah M, Mahmoud AM (2019) Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci 221:83–92
Zhao CH, Liu HQ, Cao R, Ji AL, Zhang L, Wang F, Yang RH (2012) Effects of dietary fish oil on learning function and apoptosis of hippocampal pyramidal neurons in streptozotocin-diabetic rats. Brain Res 1457:33–43
Wang J, Wang L, Zhou J, Qin A, Chen Z (2018) The protective effect of formononetin on cognitive impairment in streptozotocin (STZ)-induced diabetic mice. Biomed Pharmacother 106:1250–1257
Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY (2019) Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep 9(1):840
Stern ST, Adiseshaiah PP, Crist RM (2012) Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol 9:20
Cohignac V, Landry MJ, Boczkowski J, Lanone S (2014) Autophagy as a possible underlying mechanism of nanomaterial toxicity. Nanomaterials (Basel) 4(3):548–582
Schuhmann MK, Fluri F (2017) Effects of fullerenols on mouse brain microvascular endothelial cells. Int J Mol Sci 18(8). pii: E1783
Yamawaki H, Iwai N (2006) Cytotoxicity of water-soluble fullerene in vascular endothelial cells. Am J Physiol Cell Physiol 290(6):C1495–C1502
Johnson-Lyles DN, Peifley K, Lockett S, Neun BW, Hansen M, Clogston J, Stern ST, McNeil SE (2010) Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol Appl Pharmacol 248(3):249–258
Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R (2008) Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ 15(8):1318–1329
Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT (2007) Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol 170(1):75–86
Tian S, Lin J, Jun Zhou J, Wang X, Li Y, Ren X, Yu W, Zhong W, Xiao J, Sheng F, Chen Y, Jin C, Li S, Zheng Z, Xia B (2010) Beclin 1-independent autophagy induced by a Bcl-XL/Bcl-2 targeting compound, Z18. Autophagy 6(8):1032–1041
Gui YX, Fan XN, Wang HM, Wang G, Chen SD (2012) Glyphosate induced cell death through apoptotic and autophagic mechanisms. Neurotoxicol Teratol 34(3):344–349
Seillier M, Peuget S, Gayet O, Gauthier C, N'Guessan P, Monte M, Carrier A, Iovanna JL, Dusetti NJ (2012) TP53INP1, a tumor suppressor, interacts with LC3 and ATG8-family proteins through the LC3-interacting region (LIR) and promotes autophagy-dependent cell death. Cell Death Differ 19(9):1525–1535
Li P, Du Q, Cao Z, Guo Z, Evankovich J, Yan W, Chang Y, Shao L, Stolz DB, Tsung A, Geller DA (2012) Interferon-γ induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1). Cancer Lett 314(2):213–222
Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G (2007) Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26(10):2527–2539
Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, Hirsch JA, Stein R, Pinkas-Kramarski R (2007) Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 3(6):561–568
Wei Y, Sinha S, Levine B (2008) Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy 4(7):949–951
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27(50):6407–6418
Decuypere JP, Parys JB, Bultynck G (2012) Regulation of the autophagic bcl-2/beclin 1 interaction. Cells 1(3):284–312
Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU (2006) Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol 8(7):688–699
Wolff DJ, Barbieri CM, Richardson CF, Schuster DI, Wilson SR (2002) Trisamine C(60)-fullerene adducts inhibit neuronal nitric oxide synthase by acting as highly potent calmodulin antagonists. Arch Biochem Biophys 399(2):130–141
Martinez ZS, Castro E, Seong CS, Cerón MR, Echegoyen L, Llano M (2016) Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob Agents Chemother 60(10):5731–5741
Vance SJ, Desai V, Smith BO, Kennedy MW, Cooper A (2016) Aqueous solubilization of C60 fullerene by natural protein surfactants, latherin and ranaspumin-2. Biophys Chem 214–215:27–32
Ren SY (1852) Xu X (2015) Role of autophagy in metabolic syndrome-associated heart disease. Biochim Biophys Acta 2:225–231. https://doi.org/10.1016/j.bbadis.2014.04.029
Yamada E, Singh R (2012) Mapping autophagy on to your metabolic radar. Diabetes 61(2):272–280
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demir, E., Nedzvetsky, V.S., Ağca, C.A. et al. Pristine C60 Fullerene Nanoparticles Ameliorate Hyperglycemia-Induced Disturbances via Modulation of Apoptosis and Autophagy Flux. Neurochem Res 45, 2385–2397 (2020). https://doi.org/10.1007/s11064-020-03097-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03097-w