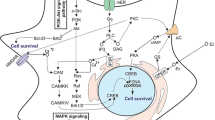
Abstract
The hippocampus-derived neuroestradiol plays a major role in neuroplasticity, independent of circulating estradiol that originates from gonads. The response of hypothalamus-pituitary regions towards the synthesis of neuroestradiol in the hippocampus is an emerging scientific concept in cognitive neuroscience. Hippocampal plasticity has been proposed to be regulated via neuroblasts, a major cellular determinant of functional neurogenesis in the adult brain. Defects in differentiation, integration and survival of neuroblasts in the hippocampus appear to be an underlying cause of neurocognitive disorders. Gonadotropin receptors and steroidogenic enzymes have been found to be expressed in neuroblasts in the hippocampus of the brain. However, the reciprocal relationship between hippocampal-specific neuroestradiol synthesis along neuroblastosis and response of pituitary based feedback regulation towards regulation of estradiol level in the hippocampus have not completely been ascertained. Therefore, this conceptual article revisits (1) the cellular basis of neuroestradiol synthesis (2) a potential relationship between neuroestradiol synthesis and neuroblastosis in the hippocampus (3) the possible involvement of aberrant neuroestradiol production with mitochondrial dysfunctions and dyslipidemia in menopause and adult-onset neurodegenerative disorders and (4) provides a hypothesis for the possible existence of the hypothalamic-pituitary-hippocampal (HPH) axis in the adult brain. Eventually, understanding the regulation of hippocampal neurogenesis by abnormal levels of neuroestradiol concentration in association with the feedback regulation of HPH axis might provide additional cues to establish a neuroregenerative therapeutic management for mood swings, depression and cognitive decline in menopause and neurocognitive disorders.

Similar content being viewed by others
Abbreviations
- 3β-HSD:
-
3-Beta-hydroxysteroid dehydrogenase
- 17β-HSD:
-
17-Beta-hydroxysteroid dehydrogenase
- AD:
-
Alzheimer’s disease
- APOE:
-
Apolipoprotein E
- BDNF:
-
Brain-derived neurotrophic factor
- CaMKII:
-
Ca2+/Calmodulin-dependent protein kinase II
- CREB:
-
cAMP response element binding protein
- ERK:
-
Extracellular signal regulated kinase
- ERα:
-
Estrogen receptor-alpha
- ERβ:
-
Estrogen receptor-beta
- FSH:
-
Follicle stimulating hormone
- GABA:
-
Gamma-aminobutyric acid
- GnRH:
-
Gonadotropin releasing hormone
- HD:
-
Huntington’s disease
- HPG:
-
Hypothalamic-pituitary–gonadal axis
- HPH:
-
Hypothalamic-pituitary-hippocampal axis
- LDL:
-
Low density lipoproteins
- LH:
-
Luteinizing hormone
- LTP:
-
Long term potentiation
- mTOR:
-
Mechanistic/mammalian target of rapamycin
- NSCs:
-
Neural stem cells
- P450scc:
-
Cholesterol side chain cleavage enzyme
- PD:
-
Parkinson’s disease
- PI3K:
-
Phosphoinositide 3-kinase
- PKB:
-
Protein kinase B
- StAR:
-
Steroidogenic acute regulatory protein
References
Cui J, Shen Y, Li R (2013) Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol Med 19:197–209. https://doi.org/10.1016/j.molmed.2012.12.007
Yaşar P, Ayaz G, User SD et al (2016) Molecular mechanism of estrogen–estrogen receptor signaling. Reprod Med Biol 16:4–20. https://doi.org/10.1002/rmb2.12006
Luine VN (2014) Estradiol and cognitive function: past, present and future. Horm Behav 66:602–618. https://doi.org/10.1016/j.yhbeh.2014.08.011
Santoro N, Randolph JF (2011) Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am 38:455–466. https://doi.org/10.1016/j.ogc.2011.05.004
Luetters C, Huang M-H, Seeman T et al (2007) Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women’s health across the nation (SWAN). J Womens Health (Larchmt) 16:331–344. https://doi.org/10.1089/jwh.2006.0057
Randolph JF, Sowers M, Bondarenko IV et al (2004) Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561. https://doi.org/10.1210/jc.2003-031183
Mason AS (1976) The menopause: the events of the menopause. R Soc Health J 96:70–71
Robinson FE, Etches RJ (1986) Ovarian steroidogenesis during follicular maturation in the domestic fowl (Gallus domesticus). Biol Reprod 35:1096–1105
Shepherd JE (2001) Effects of estrogen on congnition mood, and degenerative brain diseases. J Am Pharm Assoc (Wash) 41:221–228
Maki PM, Henderson VW (2012) Hormone therapy, dementia, and cognition: the Women’s Health Initiative ten years on. Climacteric 15:256–262. https://doi.org/10.3109/13697137.2012.660613
Rettberg JR, Dang H, Hodis HN et al (2016) Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: potential for detecting an at-Alzheimer’s risk metabolic phenotype. Neurobiol Aging 40:155–163. https://doi.org/10.1016/j.neurobiolaging.2016.01.011
Greendale GA, Derby CA, Maki PM (2011) Perimenopause and cognition. Obstet Gynecol Clin North Am 38:519–535. https://doi.org/10.1016/j.ogc.2011.05.007
Imtiaz B, Tuppurainen M, Rikkonen T et al (2017) Postmenopausal hormone therapy and Alzheimer disease. Neurology 88:1062–1068. https://doi.org/10.1212/WNL.0000000000003696
Santoro N, Epperson CN, Mathews SB (2015) Menopausal symptoms and their management. Endocrinol Metab Clin North Am 44:497–515. https://doi.org/10.1016/j.ecl.2015.05.001
Sherwin BB (2009) Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol 5:620–627. https://doi.org/10.1038/nrendo.2009.193
Cholerton B, Gleason CE, Baker LD, Asthana S (2002) Estrogen and Alzheimer’s disease: the story so far. Drugs Aging 19:405–427. https://doi.org/10.2165/00002512-200219060-00002
LeBlanc ES, Janowsky J, Chan BK, Nelson HD (2001) Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA 285:1489–1499
Dumas J, Hancur-Bucci C, Naylor M et al (2008) Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav 53:159–169. https://doi.org/10.1016/j.yhbeh.2007.09.011
Dumas J, Hancur-Bucci C, Naylor M et al (2006) Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology 31:2065–2078. https://doi.org/10.1038/sj.npp.1301042
Yaffe K, Sawaya G, Lieberburg I, Grady D (1998) Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 279:688–695
Henderson VW (2008) Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol 51:618–626. https://doi.org/10.1097/GRF.0b013e318180ba10
Henderson VW, Benke KS, Green RC et al (2005) Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry 76:103–105. https://doi.org/10.1136/jnnp.2003.024927
Simpkins JW, Perez E, Wang X et al (2009) The potential for estrogens in preventing Alzheimer’s disease and vascular dementia. Ther Adv Neurol Disord 2:31–49. https://doi.org/10.1177/1756285608100427
Gibbs RB (2000) Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116
Maki PM, Zonderman AB, Resnick SM (2001) Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry 158:227–233. https://doi.org/10.1176/appi.ajp.158.2.227
Maki PM, Dumas J (2009) Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med 27:250–259. https://doi.org/10.1055/s-0029-1216278
Lobo RA (1995) Benefits and risks of estrogen replacement therapy. Am J Obstet Gynecol 173:982–989
Lethaby A, Hogervorst E, Richards M, et al (2008) Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003122.pub2
Lavi R, Doniger GM, Simon E et al (2007) The effect of hormone replacement therapy on cognitive function in post-menopausal women. QJM 100:567–573. https://doi.org/10.1093/qjmed/hcm065
Henderson KM, Gorban AM, Boyd GS (1981) Effect of LH factors regulating ovarian cholesterol metabolism and progesterone synthesis in PMSG-primed immature rats. J Reprod Fertil 61:373–380
Kandasamy M, Aigner L (2018) Reactive neuroblastosis in huntington’s disease: a putative therapeutic target for striatal regeneration in the adult brain. Front Cell Neurosci. https://doi.org/10.3389/fncel.2018.00037
Kandasamy M, Aigner L (2018) Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders. Neural Regen Res 13:1322–1326. https://doi.org/10.4103/1673-5374.235214
Couillard-Despres S, Winner B, Schaubeck S et al (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14. https://doi.org/10.1111/j.1460-9568.2004.03813.x
Ming G-L, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. https://doi.org/10.1016/j.neuron.2011.05.001
Barha CK, Galea LAM (2010) Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta 1800:1056–1067. https://doi.org/10.1016/j.bbagen.2010.01.006
Bless EP, Yang J, Acharya KD, et al (2016) Adult neurogenesis in the female mouse hypothalamus: estradiol and high-fat diet alter the generation of newborn neurons expressing estrogen receptor α. eNeuro. https://doi.org/10.1523/ENEURO.0027-16.2016
Kretz O, Fester L, Wehrenberg U et al (2004) Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci 24:5913–5921. https://doi.org/10.1523/JNEUROSCI.5186-03.2004
Fester L, Prange-Kiel J, Jarry H, Rune GM (2011) Estrogen synthesis in the hippocampus. Cell Tissue Res 345:285–294. https://doi.org/10.1007/s00441-011-1221-7
Fester L, Brandt N, Windhorst S et al (2016) Control of aromatase in hippocampal neurons. J Steroid Biochem Mol Biol 160:9–14. https://doi.org/10.1016/j.jsbmb.2015.10.009
McCarthy MM (2009) The two faces of estradiol: effects on the developing brain. Neuroscientist 15:599–610. https://doi.org/10.1177/1073858409340924
Garcia-Segura LM, Wozniak A, Azcoitia I et al (1999) Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89:567–578
Hojo Y, Higo S, Kawato S et al (2011) Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2011.00043
Jeng S-R, Yueh W-S, Pen Y-T et al (2012) Expression of aromatase in radial glial cells in the brain of the Japanese Eel provides insight into the evolution of the cyp191a gene in actinopterygians. PLoS ONE 7:e44750. https://doi.org/10.1371/journal.pone.0044750
Mensah-Nyagan AG, Do-Rego JL, Beaujean D et al (1999) Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev 51:63–81
Janicki SC, Schupf N (2010) Hormonal influences on cognition and risk for Alzheimer disease. Curr Neurol Neurosci Rep 10:359–366. https://doi.org/10.1007/s11910-010-0122-6
Rocca WA, Grossardt BR, Shuster LT (2014) Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol 389:7–12. https://doi.org/10.1016/j.mce.2014.01.020
Terasawa E, Kenealy BP (2012) Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol 33:364–375. https://doi.org/10.1016/j.yfrne.2012.08.001
Schlinger BA, Remage-Healey L, Rensel M (2014) Establishing regional specificity of neuroestrogen action. Gen Comp Endocrinol 205:235–241. https://doi.org/10.1016/j.ygcen.2014.03.043
Plant TM (2015) The hypothalamo-pituitary-gonadal axis. J Endocrinol 226:T41–T54. https://doi.org/10.1530/JOE-15-0113
Peper JS, Brouwer RM, van Leeuwen M et al (2010) HPG-axis hormones during puberty: a study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology 35:133–140. https://doi.org/10.1016/j.psyneuen.2009.05.025
Nelson BS, Black KL, Daniel JM (2016) Circulating estradiol regulates brain-derived estradiol via actions at GnRH receptors to impact memory in ovariectomized rats. eNeuro. https://doi.org/10.1523/ENEURO.0321-16.2016
Jacobson L, Sapolsky R (1991) The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134. https://doi.org/10.1210/edrv-12-2-118
Roselli CE (2007) Brain aromatase: roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol 106:143. https://doi.org/10.1016/j.jsbmb.2007.05.014
de Bournonville C, Balthazart J, Ball GF, Cornil CA (2016) Non-ovarian aromatization is required to activate female sexual motivation in testosterone-treated ovariectomized quail. Horm Behav 83:45–59. https://doi.org/10.1016/j.yhbeh.2016.05.011
Roselli CE, Liu M, Hurn PD (2009) Brain aromatization: classical roles and new perspectives. Semin Reprod Med 27:207–217. https://doi.org/10.1055/s-0029-1216274
Velusamy T, Panneerselvam AS, Purushottam M et al (2017) Protective effect of antioxidants on neuronal dysfunction and plasticity in Huntington’s disease. Oxid Med Cell Longev 2017:3279061. https://doi.org/10.1155/2017/3279061
Periyasamy S, Sathya M, Karthick C et al (2017) Association studies of specific cholesterol related genes (APOE, LPL, and CETP) with lipid profile and memory function: a correlative study among rural and tribal population of Dharmapuri district, India. J Alzheimers Dis 60:S195–S207. https://doi.org/10.3233/JAD-170272
Gupte AA, Pownall HJ, Hamilton DJ (2015) Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res 2015:916585. https://doi.org/10.1155/2015/916585
Li J, Siegel M, Yuan M et al (2011) Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab 31:413–425. https://doi.org/10.1038/jcbfm.2010.181
McCarthy MM (2008) Estradiol and the developing brain. Physiol Rev 88:91–124. https://doi.org/10.1152/physrev.00010.2007
Tremblay JJ (2015) Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 103:3–10. https://doi.org/10.1016/j.steroids.2015.08.001
Amsterdam A, Keren-Tal I, Aharoni D et al (2003) Steroidogenesis and apoptosis in the mammalian ovary. Steroids 68:861–867
Christensen A, Bentley GE, Cabrera R et al (2012) Hormonal regulation of female reproduction. Horm Metab Res 44:587–591. https://doi.org/10.1055/s-0032-1306301
LaPolt PS, Tilly JL, Aihara T et al (1992) Gonadotropin-induced up- and down-regulation of ovarian follicle-stimulating hormone (FSH) receptor gene expression in immature rats: effects of pregnant mare’s serum gonadotropin, human chorionic gonadotropin, and recombinant FSH. Endocrinology 130:1289–1295. https://doi.org/10.1210/endo.130.3.1537292
Palermo R (2007) Differential actions of FSH and LH during folliculogenesis. Reprod Biomed Online 15:326–337
Kowalski KI, Tilly JL, Johnson AL (1991) Cytochrome P450 side-chain cleavage (P450scc) in the hen ovary I Regulation of P450scc messenger RNA levels and steroidogenesis in theca cells of developing follicles. Biol Reprod 45:955–966
Jones PB, Hsueh AJ (1982) Regulation of ovarian 3 beta-hydroxysteroid dehydrogenase activity by gonadotropin-releasing hormone and follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology 110:1663–1671. https://doi.org/10.1210/endo-110-5-1663
Sasano H, Mori T, Sasano N et al (1990) Immunolocalization of 3 beta-hydroxysteroid dehydrogenase in human ovary. J Reprod Fertil 89:743–751
Zhang Y, Word RA, Fesmire S et al (1996) Human ovarian expression of 17 beta-hydroxysteroid dehydrogenase types 1, 2, and 3. J Clin Endocrinol Metab 81:3594–3598. https://doi.org/10.1210/jcem.81.10.8855807
Stocco C (2008) Aromatase expression in the ovary: hormonal and molecular regulation. Steroids 73:473–487. https://doi.org/10.1016/j.steroids.2008.01.017
Barbieri RL (2014) The endocrinology of the menstrual cycle. Methods Mol Biol 1154:145–169. https://doi.org/10.1007/978-1-4939-0659-8_7
Hawkins SM, Matzuk MM (2008) Menstrual cycle: basic biology. Ann N Y Acad Sci 1135:10–18. https://doi.org/10.1196/annals.1429.018
Lenton EA, Sulaiman R, Sobowale O, Cooke ID (1982) The human menstrual cycle: plasma concentrations of prolactin, LH, FSH, oestradiol and progesterone in conceiving and non-conceiving women. J Reprod Fertil 65:131–139
Marques P, Skorupskaite K, George JT, Anderson RA (2000) Physiology of GNRH and gonadotropin secretion. In: Feingold KR, Anawalt B, Boyce A, et al (eds) Endotext. MDText.com, Inc., South Dartmouth
Baulieu EE, Robel P (1990) Neurosteroids: a new brain function? J Steroid Biochem Mol Biol 37:395–403
McEwen BS, Akama KT, Spencer-Segal JL et al (2012) Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci 126:4–16. https://doi.org/10.1037/a0026708
Prange-Kiel J, Jarry H, Schoen M et al (2008) Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J Cell Biol 180:417–426. https://doi.org/10.1083/jcb.200707043
Meethal SV, Liu T, Chan HW et al (2009) Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. J Neurochem 110:1014–1027. https://doi.org/10.1111/j.1471-4159.2009.06192.x
Naftolin F, Ryan KJ, Petro Z (1971) Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab 33:368–370. https://doi.org/10.1210/jcem-33-2-368
Le Goascogne C, Robel P, Gouézou M et al (1987) Neurosteroids: cytochrome P-450scc in rat brain. Science 237:1212–1215
Leblanc P, Crumeyrolle M, Latouche J et al (1988) Characterization and distribution of receptors for gonadotropin-releasing hormone in the rat hippocampus. Neuroendocrinology 48:482–488. https://doi.org/10.1159/000125053
Jennes L, Brame B, Centers A et al (1995) Regulation of hippocampal gonadotropin releasing hormone (GnRH) receptor mRNA and GnRH-stimulated inositol phosphate production by gonadal steroid hormones. Brain Res Mol Brain Res 33:104–110
Prange-Kiel J, Schmutterer T, Fester L et al (2013) Endocrine regulation of estrogen synthesis in the hippocampus? Prog Histochem Cytochem 48:49–64. https://doi.org/10.1016/j.proghi.2013.07.002
Ben-Jonathan N, Mical RS, Porter JC (1973) Superfusion of hemipituitaries with portal blood I LRF secretion in castrated and diestrous rats. Endocrinology 93:497–503. https://doi.org/10.1210/endo-93-2-497
Constantin S (2017) Progress and challenges in the search for the mechanisms of pulsatile gonadotropin-releasing hormone secretion. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2017.00180
Merchenthaler I, Görcs T, Sétáló G et al (1984) Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res 237:15–29
Skinner DC, Albertson AJ, Navratil A et al (2009) GnRH effects outside the hypothalamo-pituitary-reproductive axis. J Neuroendocrinol 21:282–292. https://doi.org/10.1111/j.1365-2826.2009.01842.x
Caraty A, Skinner DC (2008) Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid: endogenous distribution and exogenous uptake. Endocrinology 149:5227–5234. https://doi.org/10.1210/en.2007-1636
Romanelli RG, Barni T, Maggi M et al (2004) Expression and function of gonadotropin-releasing hormone (GnRH) receptor in human olfactory GnRH-secreting neurons: an autocrine GnRH loop underlies neuronal migration. J Biol Chem 279:117–126. https://doi.org/10.1074/jbc.M307955200
Casoni F, Malone SA, Belle M et al (2016) Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development 143:3969–3981. https://doi.org/10.1242/dev.139444
Chu C, Gao G, Huang W (2008) A study on co-localization of FSH and its receptor in rat hippocampus. J Mol Histol 39:49–55. https://doi.org/10.1007/s10735-007-9125-2
Reubi JC, Palacios JM, Maurer R (1987) Specific luteinizing-hormone-releasing hormone receptor binding sites in hippocampus and pituitary: an autoradiographical study. Neuroscience 21:847–856
Blair JA, Bhatta S, McGee H, Casadesus G (2015) Luteinizing hormone: evidence for direct action in the CNS. Horm Behav 76:57–62. https://doi.org/10.1016/j.yhbeh.2015.06.020
Zwain IH, Yen SS (1999) Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology 140:3843–3852. https://doi.org/10.1210/endo.140.8.6907
Porcu P, Barron AM, Frye CA et al (2016) Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol 28:12351. https://doi.org/10.1111/jne.12351
Wehrenberg U, Prange-Kiel J, Rune GM (2001) Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem 76:1879–1886
Yague JG, Wang AC-J, Janssen WGM et al (2008) Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res 1209:115–127. https://doi.org/10.1016/j.brainres.2008.02.061
Azcoitia I, Yague JG, Garcia-Segura LM (2011) Estradiol synthesis within the human brain. Neuroscience 191:139–147. https://doi.org/10.1016/j.neuroscience.2011.02.012
Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y (1998) Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. J Neurochem 71:2231–2238
Hojo Y, Hattori T-A, Enami T et al (2004) Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA 101:865–870. https://doi.org/10.1073/pnas.2630225100
Saldanha CJ, Duncan KA, Walters BJ (2009) Neuroprotective actions of brain aromatase. Front Neuroendocrinol 30:106–118. https://doi.org/10.1016/j.yfrne.2009.04.016
Remage-Healey L, Saldanha CJ, Schlinger BA (2011) Estradiol synthesis and action at the synapse: evidence for “synaptocrine” signaling. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2011.00028
Harte-Hargrove L, MacLusky NJ, Scharfman HE (2013) BDNF-estrogen interactions in hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience 239:46–66. https://doi.org/10.1016/j.neuroscience.2012.12.029
Denley MCS, Gatford NJF, Sellers KJ, Srivastava DP (2018) Estradiol and the development of the cerebral cortex: an unexpected role? Front Neurosci. https://doi.org/10.3389/fnins.2018.00245
Gatson JW, Simpkins JW, Yi KD et al (2011) Aromatase is increased in astrocytes in the presence of elevated pressure. Endocrinology 152:207–213. https://doi.org/10.1210/en.2010-0724
Liu XS, Chopp M, Zhang XG et al (2009) Gene profiles and electrophysiology of doublecortin-expressing cells in the subventricular zone after ischemic stroke. J Cereb Blood Flow Metab 29:297–307. https://doi.org/10.1038/jcbfm.2008.119
Platel J-C, Dave KA, Bordey A (2008) Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol 586:3739–3743. https://doi.org/10.1113/jphysiol.2008.155325
van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. https://doi.org/10.1038/6368
Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. https://doi.org/10.1038/386493a0
Kandasamy M, Lehner B, Kraus S et al (2014) TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J Cell Mol Med 18:1444–1459. https://doi.org/10.1111/jcmm.12298
Kandasamy M, Couillard-Despres S, Raber KA et al (2010) Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J Neuropathol Exp Neurol 69:717–728. https://doi.org/10.1097/NEN.0b013e3181e4f733
Bedos M, Portillo W, Paredes RG (2018) Neurogenesis and sexual behavior. Front Neuroendocrinol 51:68–79. https://doi.org/10.1016/j.yfrne.2018.02.004
Hutchison JB, Beyer C, Hutchison RE, Wozniak A (1997) Sex differences in the regulation of embryonic brain aromatase. J Steroid Biochem Mol Biol 61:315–322
Yue X, Lu M, Lancaster T et al (2005) Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci USA 102:19198–19203. https://doi.org/10.1073/pnas.0505203102
Fester L, Prange-Kiel J, Zhou L et al (2012) Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol 131:24–29. https://doi.org/10.1016/j.jsbmb.2011.11.010
Sasahara K, Shikimi H, Haraguchi S et al (2007) Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing purkinje cell. J Neurosci 27:7408–7417. https://doi.org/10.1523/JNEUROSCI.0710-07.2007
Smith CC, McMahon LL (2005) Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci 25:7780–7791. https://doi.org/10.1523/JNEUROSCI.0762-05.2005
Srivastava DP, Woolfrey KM, Penzes P (2013) Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev 65:1318–1350. https://doi.org/10.1124/pr.111.005272
Spencer JL, Waters EM, Romeo RD et al (2008) Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29:219–237. https://doi.org/10.1016/j.yfrne.2007.08.006
Liu F, Day M, Muñiz LC et al (2008) Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11:334–343. https://doi.org/10.1038/nn2057
Kumar A, Bean LA, Rani A et al (2015) Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice. Hippocampus 25:1556–1566. https://doi.org/10.1002/hipo.22475
Adams MM, Shah RA, Janssen WG, Morrison JH (2001) Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA 98:8071–8076. https://doi.org/10.1073/pnas.141215898
Morgan TE, Finch CE (2015) Astrocytic estrogen receptors and impaired neurotrophic responses in a rat model of perimenopause. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2015.00179
Zhou J, Zhang H, Cohen RS, Pandey SC (2005) Effects of estrogen treatment on expression of BDNF and CREB expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology 81:294–310. https://doi.org/10.1159/000088448
Fortress AM, Fan L, Orr PT et al (2013) Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem 20:147–155. https://doi.org/10.1101/lm.026732.112
O’Neill EE, Blewett AR, Loria PM, Greene GL (2008) Modulation of αCaMKII signaling by rapid ERα action. Brain Res 1222:1–17. https://doi.org/10.1016/j.brainres.2008.05.036
Sawai T, Bernier F, Fukushima T et al (2002) Estrogen induces a rapid increase of calcium-calmodulin-dependent protein kinase II activity in the hippocampus. Brain Res 950:308–311
Nakagawa S, Kim J-E, Lee R et al (2002) Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 22:3673–3682. https://doi.org/10.1523/JNEUROSCI.22-09-03673.2002
Ortega-Martínez S (2015) A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2015.00046
Ka M, Condorelli G, Woodgett JR, Kim W-Y (2014) mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development 141:4076–4086. https://doi.org/10.1242/dev.108282
Hugo J, Ganguli M (2014) Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 30:421–442. https://doi.org/10.1016/j.cger.2014.04.001
Prince M, Ali G-C, Guerchet M et al (2016) Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. https://doi.org/10.1186/s13195-016-0188-8
Matthews FE, Brayne C, Lowe J et al (2009) Epidemiological pathology of dementia: attributable-risks at death in the medical research council cognitive function and ageing study. PLOS Med 6:e1000180. https://doi.org/10.1371/journal.pmed.1000180
Lin KA, Choudhury KR, Rathakrishnan BG et al (2015) Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 1:103–110. https://doi.org/10.1016/j.trci.2015.07.001
Brinton RD, Yao J, Yin F et al (2015) Perimenopause as a neurological transition state. Nat Rev Endocrinol 11:393–405. https://doi.org/10.1038/nrendo.2015.82
Winner B, Winkler J (2015) Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol 7:a021287. https://doi.org/10.1101/cshperspect.a021287
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. https://doi.org/10.1126/science.aag2590
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. https://doi.org/10.1038/nature05292
Atwood CS, Meethal SV, Liu T et al (2005) Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol 64:93–103
Short RA, Bowen RL, O’Brien PC, Graff-Radford NR (2001) Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 76:906–909. https://doi.org/10.4065/76.9.906
Meethal SV, Smith MA, Bowen RL, Atwood CS (2005) The gonadotropin connection in Alzheimer’s disease. Endocrine 26:317–326. https://doi.org/10.1385/ENDO:26:3:317
Li R, Cui J, Shen Y (2014) Brain sex matters: estrogen in cognition and Alzheimer’s disease. Mol Cell Endocrinol 389:13–21. https://doi.org/10.1016/j.mce.2013.12.018
Tang MX, Jacobs D, Stern Y et al (1996) Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348:429–432. https://doi.org/10.1016/S0140-6736(96)03356-9
Chu MC, Rath KM, Huie J, Taylor HS (2003) Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Hum Reprod 18:1570–1573
Barañao JL, Hammond JM (1986) FSH increases the synthesis and stores of cholesterol in porcine granulosa cells. Mol Cell Endocrinol 44:227–236
Rosati F, Sturli N, Cungi MC et al (2011) Gonadotropin-releasing hormone modulates cholesterol synthesis and steroidogenesis in SH-SY5Y cells. J Steroid Biochem Mol Biol 124:77–83. https://doi.org/10.1016/j.jsbmb.2011.01.012
Miller WL, Bose HS (2011) Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 52:2111–2135. https://doi.org/10.1194/jlr.R016675
Palmisano BT, Zhu L, Stafford JM (2017) Estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol 1043:227–256. https://doi.org/10.1007/978-3-319-70178-3_12
Phan BAP, Toth PP (2014) Dyslipidemia in women: etiology and management. Int J Womens Health 6:185–194. https://doi.org/10.2147/IJWH.S38133
Moroney JT, Tang MX, Berglund L et al (1999) Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA 282:254–260
Liu J-P, Tang Y, Zhou S et al (2010) Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol Cell Neurosci 43:33–42. https://doi.org/10.1016/j.mcn.2009.07.013
Vance JE (2012) Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 5:746–755. https://doi.org/10.1242/dmm.010124
de Chaves EP, Narayanaswami V (2008) Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol 3:505–530
Sowers MR, Zheng H, McConnell D et al (2008) Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab 93:3958–3964. https://doi.org/10.1210/jc.2008-0482
Song Y, Wang E-S, Xing L-L et al (2016) Follicle-stimulating hormone induces postmenopausal dyslipidemia through inhibiting hepatic cholesterol metabolism. J Clin Endocrinol Metab 101:254–263. https://doi.org/10.1210/jc.2015-2724
Ngo Sock ET, Côté I, Mentor JS et al (2013) Ovariectomy stimulates hepatic fat and cholesterol accumulation in high-fat diet-fed rats. Horm Metab Res 45:283–290. https://doi.org/10.1055/s-0032-1329964
Burger HG, Hale GE, Robertson DM, Dennerstein L (2007) A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update 13:559–565. https://doi.org/10.1093/humupd/dmm020
Butler L, Santoro N (2011) The reproductive endocrinology of the menopausal transition. Steroids 76:627–635. https://doi.org/10.1016/j.steroids.2011.02.026
Su HI, Freeman EW (2009) Hormone changes associated with the menopausal transition. Minerva Ginecol 61:483–489
Grisendi V, Spada E, Argento C et al (2014) Age-specific reference values for serum FSH and estradiol levels throughout the reproductive period. Gynecol Endocrinol 30:451–455. https://doi.org/10.3109/09513590.2014.893572
Saxena AR, Seely EW (2012) Luteinizing hormone correlates with adrenal function in postmenopausal women. Menopause 19:1280–1283. https://doi.org/10.1097/gme.0b013e31825540c4
Liu T, Wimalasena J, Bowen RL, Atwood CS (2007) Luteinizing hormone receptor mediates neuronal pregnenolone production via up-regulation of steroidogenic acute regulatory protein expression. J Neurochem 100:1329–1339. https://doi.org/10.1111/j.1471-4159.2006.04307.x
Casadesus G, Webber KM, Atwood CS et al (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762:447–452. https://doi.org/10.1016/j.bbadis.2006.01.008
Rodrigues MA, Verdile G, Foster JK et al (2008) Gonadotropins and cognition in older women. J Alzheimer’s Dis 13:267–274. https://doi.org/10.3233/JAD-2008-13304
Lin J, Li X, Yuan F et al (2010) Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol 69:253–261. https://doi.org/10.1097/NEN.0b013e3181d072cf
Webber KM, Casadesus G, Zhu X et al (2006) The cell cycle and hormonal fluxes in Alzheimer disease: a novel therapeutic target. Curr Pharm Des 12:691–697
Prange-Kiel J, Dudzinski DA, Pröls F et al (2016) Aromatase expression in the hippocampus of AD patients and 5xFAD mice. Neural Plast 2016:9802086. https://doi.org/10.1155/2016/9802086
Jin K, Peel AL, Mao XO et al (2004) Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA 101:343–347. https://doi.org/10.1073/pnas.2634794100
Rodríguez JJ, Jones VC, Tabuchi M et al (2008) Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS ONE 3:e2935. https://doi.org/10.1371/journal.pone.0002935
West NA, Haan MN (2009) Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. J Gerontol A Biol Sci Med Sci 64A:103–109. https://doi.org/10.1093/gerona/gln006
Lovejoy J, Champagne C, de Jonge L et al (2008) Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32:949–958. https://doi.org/10.1038/ijo.2008.25
Piché M-E, Weisnagel SJ, Corneau L et al (2005) Contribution of abdominal visceral obesity and insulin resistance to the cardiovascular risk profile of postmenopausal women. Diabetes 54:770–777
Kim J, Choi K-H, Cho S-G et al (2019) Association of muscle and visceral adipose tissues with the probability of Alzheimer’s disease in healthy subjects. Sci Rep 9:949. https://doi.org/10.1038/s41598-018-37244-9
Raschpichler M, Straatman K, Schroeter ML et al (2013) Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open. https://doi.org/10.1136/bmjopen-2012-001915
Rebuffé-Scrive M, Eldh J, Hafström LO, Björntorp P (1986) Metabolism of mammary, abdominal, and femoral adipocytes in women before and after menopause. Metab Clin Exp 35:792–797
Liu P, Ji Y, Yuen T et al (2017) Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546:107–112. https://doi.org/10.1038/nature22342
Zhao L, Zhu C, Chen Y et al (2018) LH/FSH ratio is associated with visceral adipose dysfunction in Chinese women older than 55. Front Endocrinol (Lausanne) 9:419. https://doi.org/10.3389/fendo.2018.00419
Klinge CM (2008) Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem 105:1342–1351. https://doi.org/10.1002/jcb.21936
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13:566–578. https://doi.org/10.1038/nrm3412
Simpkins JW, Yang S, Sarkar SN, Pearce V (2008) Estrogen actions on mitochondria-physiological and pathological implications. Mol Cell Endocrinol 290:51–59. https://doi.org/10.1016/j.mce.2008.04.013
Miller WL, Auchus RJ (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151. https://doi.org/10.1210/er.2010-0013
Arakane F, Kallen CB, Watari H et al (1998) The mechanism of action of steroidogenic acute regulatory protein (StAR) StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem 273:16339–16345. https://doi.org/10.1074/jbc.273.26.16339
Kandasamy M, Reilmann R, Winkler J et al (2011) Transforming growth factor-beta signaling in the neural stem cell niche: a therapeutic target for Huntington’s disease. Neurol Res Int 2011:124256. https://doi.org/10.1155/2011/124256
Papadopoulos V, Miller WL (2012) Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab 26:771–790. https://doi.org/10.1016/j.beem.2012.05.002
Fowler CD, Liu Y, Wang Z (2008) Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev 57:342–351. https://doi.org/10.1016/j.brainresrev.2007.06.011
Chan M, Chow C, Hamson DK et al (2014) Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. J Neuroendocrinol 26:386–399. https://doi.org/10.1111/jne.12159
Torner L, Karg S, Blume A et al (2009) Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J Neurosci 29:1826–1833. https://doi.org/10.1523/JNEUROSCI.3178-08.2009
Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19:5792–5801
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85. https://doi.org/10.1186/1750-1326-6-85
Villa A, Vegeto E, Poletti A, Maggi A (2016) Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev 37:372–402. https://doi.org/10.1210/er.2016-1007
James K, Bhartiya D, Ganguly R, et al (2018) Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium. J Ovarian Res https://doi.org/10.1186/s13048-018-0454-4
Acknowledgements
MK has been supported by the Faculty Recharge Programme, University Grants Commission (UGC-FRP), New Delhi, India. MK would like to greatly acknowledge a start-up Grant from UGC-FRP, a research Grant (EEQ/2016/000639) and an Early Career Research Award (ECR/2016/000741) from DST-SERB, New Delhi, India. AY has been supported as JRF from DST SERB-EEQ/2016/000639. SAR has been supported as JRF from the DBT, India. CP thank DST-SERB, New Delhi, India for financial support (EMR/2017/003670). MME is highly acknowledging the support given by SQU in the form of an internal Grant (IG/AGR/FOOD/17/02). The authors acknowledge UGC-SAP, DST-FIST and PURSE for the infrastructure of the Department of Animal Science and Department of Biochemistry, Bharathidasan University.
Author information
Authors and Affiliations
Contributions
MK conceived the present idea, hypothesis and generated the illustration. RKR, PAGP, MK and MA further developed the hypothesis and performed the literature search and made initial draft. MK, RKR, PAGP, SAR, AY, KB, CP, SS, AM, MME, MA contributed to the revision of article, made critical comments and suggestions. All authors discussed the content and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kandasamy, M., Radhakrishnan, R.K., Poornimai Abirami, G.P. et al. Possible Existence of the Hypothalamic-Pituitary-Hippocampal (HPH) Axis: A Reciprocal Relationship Between Hippocampal Specific Neuroestradiol Synthesis and Neuroblastosis in Ageing Brains with Special Reference to Menopause and Neurocognitive Disorders. Neurochem Res 44, 1781–1795 (2019). https://doi.org/10.1007/s11064-019-02833-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02833-1