Abstract
The mechanistic target of rapamycin (mTOR) has been demonstrated to mediate pain-related aversion induced by formalin in the rostral anterior cingulate cortex (rACC). However, it remains unclear the signaling pathways and regulatory proteins involved. In the rACC, brain-derived neurotrophic factor (BDNF), an activity-dependent neuromodulator, has been shown to play a role in the development and persistence of chronic pain. In this study, we used a rat formalin-induced inflammatory pain model to demonstrate BDNF up-regulation in the rACC. Stimulation with exogenous BDNF up-regulated mTOR, whilst cyclotraxin B (CTX-B), a tropomyosin receptor kinase B (TrkB) antagonist, down-regulated mTOR. Our results suggest BDNF could activate an mTOR signaling pathway. Subsequently, we used formalin-induced conditioned place avoidance (F-CPA) training in rat models to investigate if mTOR activation was required for pain-related aversion. We demonstrated that BDNF/mTOR signaling could activate the NMDA receptor subunit episilon-2 (NR2B), which is required for F-CPA. Our results reveal that BDNF activates mTOR to up-regulate NR2B expression, which is required for inflammatory pain-related aversion in the rACC of rats.

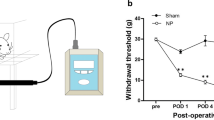
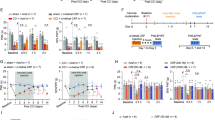
Adapted from Jones et al. 2008. (Color figure online)







Similar content being viewed by others
References
Rhudy JL, Meagher MW (2000) Fear and anxiety: divergent effects on human pain thresholds. Pain 84(1):65–75
Al Absi M, Rokke PD (1991) Can anxiety help us tolerate pain? Pain 46(1):43–51
Lu B et al (2016) Inhibition of mammalian target of rapamycin activation in the rostral anterior cingulate cortex attenuates pain-related aversion in rats. Behav Brain Res 310:51–58
Jeon D et al (2010) Observational fear learning involves affective pain system and Cav12 Ca2+ channels in ACC. Nat Neurosci 13(4):482–488
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288(5472):1769–1772
Costa-Mattioli M et al (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61(1):10–26
Sarbassov DD, Ali SM, Sabatini DM (2005) Growing roles for the mTOR pathway. Curr Opin Cell Biol 17(6):596–603
Jaworski J, Sheng M (2006) The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol 34(3):205–219
Ruvinsky I, Meyuhas O (2006) Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31(6):342–348
Tang SJ et al (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 99(1):467–472
Cammalleri M et al (2003) Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA 100(24):14368–14373
Asante CO, Wallace VC, Dickenson AH (2009) Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain 5:27
Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270(5236):593–598
Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2(1):24–32
Santos AR, Comprido D, Duarte CB (2010) Regulation of local translation at the synapse by BDNF. Prog Neurobiol 92(4):505–516
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23
Yoshii A, Constantine-Paton M (2010) Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 70(5):304–322
Takei N et al (2004) Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 24(44):9760–9769
Thibault K et al (2014) BDNF-dependent plasticity induced by peripheral inflammation in the primary sensory and the cingulate cortex triggers cold allodynia and reveals a major role for endogenous BDNF as a tuner of the affective aspect of pain. J Neurosci 34(44):14739–14751
Zhang L et al (2016) Brain-derived neurotrophic factor (BDNF) in the rostral anterior cingulate cortex (rACC) contributes to neuropathic spontaneous pain-related aversion via NR2B receptors. Brain Res Bull 127:56–65
Zhao MG et al (2006) Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 26(35):8923–8930
Wu LJ et al (2005) Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 25(48):11107–11116
Schratt GM et al (2004) BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24(33):7366–7377
Xiao X et al (2013) Estrogen in the anterior cingulate cortex contributes to pain-related aversion. Cereb Cortex 23(9):2190–2203
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Chaplan SR et al (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Gao YJ et al (2004) Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110(1–2):343–353
Dash PK, Orsi SA, Moore AN (2006) Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci 26(31):8048–8056
Fu KY, Light AR, Maixner W (2001) Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain 2(1):2–11
Fu KY et al (1999) Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res 825(1–2):59–67
Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10(9):895–926
Gottmann K, Mittmann T, Lessmann V (2009) BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res 199(3–4):203–234
Geranton SM et al (2009) A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci 29(47):15017–15027
Jiang F et al (2014) Activation of mammalian target of rapamycin contributes to pain nociception induced in rats by BmK I, a sodium channel-specific modulator. Neurosci Bull 30(1):21–32
Slipczuk L et al (2009) BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE 4(6):e6007
Inamura N, Nawa H, Takei N (2005) Enhancement of translation elongation in neurons by brain-derived neurotrophic factor: implications for mammalian target of rapamycin signaling. J Neurochem 95(5):1438–1445
Liang L et al (2013) mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res 1513:17–25
Gupta VK et al (2013) Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci 49(1):96–104
Cazorla M et al (2010) Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice. PLoS ONE 5(3):e9777
Gupta V et al (2014) Brain derived neurotrophic factor is involved in the regulation of glycogen synthase kinase 3beta (GSK3beta) signalling. Biochem Biophys Res Commun 454(3):381–386
Gupta V et al (2014) BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochim Biophys Acta 1842(9):1567–1578
Chitranshi N et al (2016) Molecular determinants and interaction data of cyclic peptide inhibitor with the extracellular domain of TrkB receptor. Data Brief 6:776–782
Heppenstall PA, Lewin GR (2001) BDNF but not NT-4 is required for normal flexion reflex plasticity and function. Proc Natl Acad Sci USA 98(14):8107–8112
Kerr BJ et al (1999) Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci 19(12):5138–5148
Lin YT et al (2011) Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation 8:126
Caldeira MV et al (2007) BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci 35(2):208–219
Geng SJ et al (2010) Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol 222(2):256–266
Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33(2):67–75
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
May A (2008) Chronic pain may change the structure of the brain. Pain 137(1):7–15
Norsted Gregory E et al (2010) Mammalian target of rapamycin in spinal cord neurons mediates hypersensitivity induced by peripheral inflammation. Neuroscience 169(3):1392–1402
Lyu D et al (2013) The mTOR signaling pathway regulates pain-related synaptic plasticity in rat entorhinal-hippocampal pathways. Mol Pain 9:64
Acknowledgements
The present work was supported by the Natural Science Foundation of Shandong Province (2013ZRB14282), the Key Researchand Development Project of Shandong Province (2014GGH218033) and the National Natural Science Foundation of China (NSFC, 30872433).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Ji, F., Wang, G. et al. BDNF Activates mTOR to Upregulate NR2B Expression in the Rostral Anterior Cingulate Cortex Required for Inflammatory Pain-Related Aversion in Rats. Neurochem Res 43, 681–691 (2018). https://doi.org/10.1007/s11064-018-2470-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2470-6