Abstract
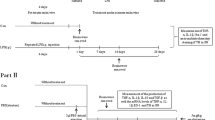
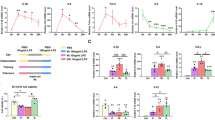
Neuroinflammation is an important pathogenesis of Parkinson’s disease (PD). The peripheral immune system could produce profound effects on central immunities. The peripheral blood monocyte (PBM) immune tolerance is the refractoriness of immune system to avoid overactive peripheral inflammation. The PBM are also actively involved in central immune activities. There is evidence implying the probable failure of immune tolerance and impairment of CD200/CD200R signaling in PD patients. Here we aimed to explore the effects of PBM tolerance in peripheral LPS-induced neuroinflammation as well as the specific roles of CD200/CD200R pathway in PBM tolerance. We found that repeated intraperitoneal administration of 0.3 mg/kg LPS was able to induce the PBM tolerance. PBM tolerance reduced peripheral LPS-induced elevation of serum TNF-α, IL-1β expression and TLR4 expression in PBM. PBM tolerance and PBM depletion alleviated peripheral LPS-induced neuroinflammation demonstrated by reduced proinflammatory cytokines in brain and blocked microglia activation. The CD200R expression in PBM was upregulated in PBM tolerance group after intraperitoneal administration of high-dose LPS in vivo and the blockade of CD200/CD200R interaction induced the failure of PBM tolerance in vitro. These results suggested the PBM tolerance could attenuate the peripheral LPS-induced neuroinflammation via upregulating the CD200R expression and the CD200/CD200R signaling played a key role in PBM tolerance. Effective regulation of the PBM in periphery may be a potential way to limit neuroinflammation while the CD200R on PBM could be used as a potential therapeutic target to alleviate neuroinflammation.









Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- PBM:
-
Peripheral blood monocyte
- LPS:
-
Lipopolysaccharides
- SN:
-
Substantia nigra
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- BBB:
-
Blood brain barrier
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- TH:
-
Tyrosine hydroxylase
- McAb:
-
Monoclonal antibody
- PcAb:
-
Polyclonal antibody
- ANOVA:
-
Analysis of variance
References
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150:963–976
Schreuder L, Eggen BJ, Biber K, Schoemaker RG, Laman JD, de Rooij SE (2017) Pathophysiological and behavioral effects of systemic inflammation in aged and diseased rodents with relevance to delirium: a systematic review. Brain Behav Immun 62:362–381
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D (2015) Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflamm 12:114
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462
Machado A, Herrera AJ, Venero JL, Santiago M, De Pablos RM, Villarán RF, Espinosa-Oliva AM, Argüelles S, Sarmiento M, Delgado-Cortés MJ, Mauriño R, Cano J (2011) Peripheral inflammation increases the damage in animal models of nigrostriatal dopaminergic neurodegeneration: possible implication in Parkinson’s disease incidence. Parkinsons Dis 2011:393769.
Sung YF, Liu FC, Lin CC, Lee JT, Yang FC, Chou YC, Lin CL, Kao CH, Lo HY, Yang TY (2016) Reduced risk of Parkinson disease in patients with rheumatoid arthritis: a nationwide population-based study. Mayo Clin Proc 91:1346–1353
Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH (2009) Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology 73:1462–1468
Italiani P, Boraschi D (2014) From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 5:514.
Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernández-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U (2001) Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 7:1356–1361
Prinz M, Priller J, Sisodia SS, Ransohoff RM (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14:1227–1235
Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF (2015) Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289:429–442
Minogue AM (2017) Role of infiltrating monocytes/macrophages in acute and chronic neuroinflammation: effects on cognition, learning and affective behaviour. Prog Neuropsychopharmacol Biol Psychiatry 79:15–18
Cao JJ, Li KS, Shen YQ (2011) Activated immune cells in Parkinson’s disease. J Neuroimmune Pharmacol 6:323–329
López-Collazo E, del Fresno C (2013) Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care 17:242
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6:e28032
Holmannová D, Kolácková M, Kondélková K, Kunes P, Krejsek J, Andrýs C (2012) CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part I: CD200/CD200R structure, activation, and function. Acta Med 55:12–17
Ryqiel TP, Meyaard L (2012) CD200R signaling in tumor tolerance and inflammation: a tricky balance. Curr Opin Immunol 24:233–238
Luo XG, Zhang JJ, Zhang CD, Liu R, Zheng L, Wang XJ, Chen SD, Ding JQ (2010) Altered regulation of CD200 receptor in monocyte-derived macrophages from individuals with Parkinson’s disease. Neurochem Res 35:540–547
Xie X, Luo X, Liu N, Li X, Lou F, Zheng Y, Ren Y (2017) Monocytes, microglia and CD200-CD200R1 signaling are essential in the transmission of inflammation from the periphery to the central nervous system. J Neurochem 141:222–235
Kim SJ, Kim HM (2017) Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD14. BMB Rep 50:55–57
Li J, Csakai A, Jin J, Zhang F, Yin H (2016) Therapeutic developments targeting toll-like receptor-4-mediated neuroinflammation. ChemMedChem 11:154–165
Chen K, Geng S, Yuan R, Diao N, Upchurch Z, Li L (2015) Super-low dose endotoxin pre-conditioning exacerbates sepsis mortality. EBioMedicine 2:324–333.
Yang NB, Ni SL, Li SS, Zhang SN, Hu DP, Lu MQ (2015) Endotoxin tolerance alleviates experimental acute liver failure via inhibition of high mobility group box 1. Int J Clin Exp Pathol 8:9062–9071
Shi DW, Zhang J, Jiang HN, Tong CY, Gu GR, Ji Y, Summah H, Qu JM (2011) LPS pretreatment ameliorates multiple organ injuries and improves survival in a murine model of polymicrobial sepsis. Inflamm Res 60:841–849
Rios EC, Soriano FG, Olah G, Gerö D, Szczesny B, Szabo C (2016) Hydrogen sulfide modulates chromatin remodeling and inflammatory mediator production in response to endotoxin, but does not play a role in the development of endotoxin tolerance. J Inflamm 13:10.
van Rooijen N, Sanders A, van den Berg TK (1996) Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods 193:93–99
Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD (1995) The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia 15:437–446
Walker DG, Lue LF (2015) Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther 7:56.
Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP (2017) NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med 214:1351–1370
Wilkinson BL, Landreth GE (2006) The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflamm 3:30
Bagyinszky E, Giau VV, Shim K, Suk K, An SSA, Kim S (2017) Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J Neurol Sci 376:242–254
Teeling JL, Perry VH (2009) Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience 158:1062–1073
Turrin NP, Rivest S (2004) Unraveling the molecular details involved in the intimate link between the immune and neuroendocrine systems. Exp Biol Med 229:996–1006
Waschbisch A, Schröder S, Schraudner D, Sammet L, Weksler B, Melms A, Pfeifenbring S, Stadelmann C, Schwab S, Linker RA (2016) Pivotal role for CD16+ monocytes in immune surveillance of the central nervous system. J Immunol 196:1558–1567
Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M (2005) Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci 25:11125–11132
Kim WK, Avarez X, Williams K (2005) The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res 8:107–115
González H, Contreras F, Pacheco R (2015) Regulation of the neurodegenerative process associated to Parkinson’s disease by CD4+ T-cells. J Neuroimmune Pharmacol 10:561–575
Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP (2004) Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke 35:2576–2581
Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF (2009) Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol 215:5–19
Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD (2006) Regulation of myeloid cell function through the CD200 receptor. J Immunol 176:191–199
Gorczynski RM, Chen Z, Clark DA, Kai Y, Lee L, Nachman J, Wong S, Marsden P (2004) Structural and functional heterogeneity in the CD200R family of immunoregulatory molecules and their expression at the feto-maternal interface. Am J Reprod Immunol 52:147–163
Acknowledgements
This work was supported by the National Natural Science Foundation of China [No. 81371421].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Xia, L., Xie, X., Liu, Y. et al. Peripheral Blood Monocyte Tolerance Alleviates Intraperitoneal Lipopolysaccharides-Induced Neuroinflammation in Rats Via Upregulating the CD200R Expression. Neurochem Res 42, 3019–3032 (2017). https://doi.org/10.1007/s11064-017-2334-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2334-5