Abstract
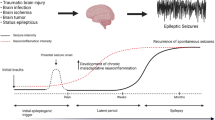
The need to find measures that reliably predict the onset of epilepsy after injurious events or how the patient will respond to anti-seizure drugs led to intensive pre-clinical and clinical research to discover non-invasive biomarkers that could increase the sensitivity of existing clinical indicators. The use of experimental models of epileptogenesis and of drug-resistance is instrumental to select the most promising approaches to explore such biomarkers in the pre-clinical setting for further clinical validation. The approaches most frequently used to find clinically useful biomarkers of epileptogenesis include molecular brain imaging, EEG signal analysis and the measure of soluble molecules in biofluids which may reflect brain intrinsic events involved in epilepsy development. Among those, we focused our attention on proton magnetic resonance imaging (1H-MRS)-based analysis of astrocytic activation, and related blood biomarkers, since this cell population appears to be pivotally involved in various epileptogenesis processes triggered by differing insults. Moreover, we also investigated behavioral biomarkers by focusing on cognitive dysfunctions since this deficit represents a typical co-morbidity in epilepsy which may manifest even before the onset of spontaneous seizures. In this review article, we will report our recently published evidence supporting the utility of measuring astrocyte activation, the soluble molecules they release, and the associated cognitive deficits during epileptogenesis for early stratification of animals developing epilepsy. We will discuss the potential clinical translation of our findings for enriching the patient population in preventive clinical trials designed to study anti-epileptogenic treatments.

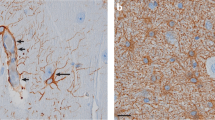
This figure has been adapted from original figures published in Ref. [39]

Similar content being viewed by others
References
Pitkanen A, Engel J (2014) Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 11:231–241
Gorter JA, van Vliet EA, Aronica E et al (2006) Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci 26:11083–11110
Pitkanen A, Lukasiuk K (2011) Molecular biomarkers of epileptogenesis. Biomark Med 5:629–633
Pitkanen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10:173–186
Henshall DC, Hamer HM, Pasterkamp RJ et al (2016) MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol 15:1368–1376
Depaulis A, David O, Charpier S (2016) The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J Neurosci Methods 260:159–174
Pennacchio LA, Bouley DM, Higgins KM et al (1998) Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nat Genet 20:251–258
Oakley JC, Kalume F, Catterall WA (2011) Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia 52(Suppl 2):59–61
Devinsky O, Vezzani A, Najjar S et al (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184
Robel S, Buckingham SC, Boni JL et al (2015) Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 35:3330–3345
Ortinski PI, Dong J, Mungenast A et al (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–591
Wang N, Mi X, Gao B et al (2015) Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience 287:144–156
Ito S, Ogiwara I, Yamada K et al (2013) Mouse with Nav1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis 49:29–40
Marques-Carneiro JE, Faure J-B, Barbelivien A et al (2016) Subtle alterations in memory systems and normal visual attention in the GAERS model of absence epilepsy. Neuroscience 316:389–401
Kleen JK, Scott RC, Lenck-Santini PP, Holmes GL (2012) Cognitive and Behavioral Co-Morbidities of Epilepsy [Internet]. Jaspers Basic Mech. Epilepsies 4th edition
Pitkanen A, McIntosh TK (2006) Animal models of post-traumatic epilepsy. J Neurotrauma 23:241–261
Pitkänen A, Roivainen R, Lukasiuk K (2015) Development of epilepsy after ischaemic stroke. Lancet Neurol 15:185–197
Löscher W (2011) Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20:359–368
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145
Lehnardt S (2010) Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia 58:253–263
Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7:194–206
Aronica E, Ravizza T, Zurolo E, Vezzani A (2012) Astrocyte immune response in epilepsy. Glia 60:1258–1268
Boer K, Spliet WG, van Rijen PC et al (2006) Evidence of activated microglia in focal cortical dysplasia. J Neuroimmunol 173:188–195
Ravizza T, Boer K, Redeker S et al (2006) The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis 24:128–143
Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298
Kumlien E, Bergstrom M, Lilja A et al (1995) Positron emission tomography with [11C] deuterium-deprenyl in temporal lobe epilepsy. Epilepsia 36:712–721
Kumlien E, Hilton-Brown P, Spannare B, Gillberg PG (1992) In vitro quantitative autoradiography of [3H]-L-deprenyl and [3H]-PK 11195 binding sites in human epileptic hippocampus. Epilepsia 33:610–617
Kumlien E, Nilsson A, Hagberg G et al (2001) PET with 11C-deuterium-deprenyl and 18F-FDG in focal epilepsy. Acta Neurol Scand 103:360–366
Gershen LD, Zanotti-Fregonara P, Dustin IH et al (2015) Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol 72:882–888
Butler T, Li Y, Tsui W et al (2016) Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia 57:e191–e194
Hirvonen J, Kreisl WC, Fujita M et al (2012) Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med 53:234–240
Haik S, Galanaud D, Linguraru MG et al (2008) In vivo detection of thalamic gliosis: a pathoradiologic demonstration in familial fatal insomnia. Arch Neurol 65:545–549
Mader I, Rauer S, Gall P, Klose U (2008) (1)H MR spectroscopy of inflammation, infection and ischemia of the brain. Eur J Radiol 67:250–257
Mizuno S, Takahashi Y, Kato Z et al (2000) Magnetic resonance spectroscopy of tubers in patients with tuberous sclerosis. Acta Neurol Scand 102:175–178
Turkdogan-Sozuer D, Ozek MM, Sav A et al (2000) Serial MRI and MRS studies with unusual findings in Rasmussen’s encephalitis. Eur Radiol 10:962–966
Hammen T, Hildebrandt M, Stadlbauer A et al (2008) Non-invasive detection of hippocampal sclerosis: correlation between metabolite alterations detected by (1)H-MRS and neuropathology. NMR Biomed 21:545–552
Filibian M, Frasca A, Maggioni D et al (2012) In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 53:1907–1916
Pascente R, Frigerio F, Rizzi M et al (2016) Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol Dis 93:146–155
Ravizza T, Gagliardi B, Noé F et al (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29:142–160
Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu D-G (2002) Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res 51:93–107
Amhaoul H, Hamaide J, Bertoglio D et al (2015) Brain inflammation in a chronic epilepsy model: evolving pattern of the translocation protein during epileptogenesis. Neurobiol Dis 82:526–539
Brackhan M, Bascunana P, Postema JM et al (2016) Serial quantitative TSPO-targeted PET reveals peak microglial activation up to 2 weeks after an epileptogenic brain insult. J Nucl Med 57:1302–1308
Wu Y, Pearce PS, Rapuano A et al (2015) Metabolic changes in early poststatus epilepticus measured by MR spectroscopy in rats. J Cereb Blood Flow Metab 35:1862–1870
Shapiro LA, Wang L, Ribak CE (2008) Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia 49(Suppl 2):33–41
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Sankar R, Shin D, Mazarati AM et al (2000) Epileptogenesis after status epilepticus reflects age- and model-dependent plasticity. Ann Neurol 48:580–589
Roch C, Leroy C, Nehlig A, Namer IJ (2002) Magnetic resonance imaging in the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia 43:325–335
Leroy C, Pierre K, Simpson IA et al (2011) Temporal changes in mRNA expression of the brain nutrient transporters in the lithium-pilocarpine model of epilepsy in the immature and adult rat. Neurobiol Dis 43:588–597
Dubé C, Boyet S, Marescaux C, Nehlig A (2001) Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol 167:227–241
Sayin U, Sutula TP, Stafstrom CE (2004) Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia 45:1539–1548
Chauviere L, Rafrafi N, Thinus-Blanc C et al (2009) Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci 29:5402–5410
Hort J, Brozek G, Komarek V et al (2000) Interstrain differences in cognitive functions in rats in relation to status epilepticus. Behav Brain Res 112:77–83
Kubova H, Mares P, Suchomelova L et al (2004) Status epilepticus in immature rats leads to behavioural and cognitive impairment and epileptogenesis. Eur J Neurosci 19:3255–3265
Cilio MR, Sogawa Y, Cha BH et al (2003) Long-term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia 44:518–528
Liu Z, Gatt A, Werner SJ et al (1994) Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy Res 19:191–204
Hermann B, Jones J, Sheth R et al (2006) Children with new-onset epilepsy: neuropsychological status and brain structure. Brain 129:2609–2619
Oostrom KJ, Smeets-Schouten A, Kruitwagen CL et al (2003) Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”—a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 112:1338–1344
Witt JA, Helmstaedter C (2015) Cognition in the early stages of adult epilepsy. Seizure 26:65–68
Austin JK, Harezlak J, Dunn DW et al (2001) Behavior problems in children before first recognized seizures. Pediatrics 107:115–122
Berg AT, Smith SN, Frobish D et al (2005) Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol 47:749–753
Barkas L, Redhead E, Taylor M et al (2012) Fluoxetine restores spatial learning but not accelerated forgetting in mesial temporal lobe epilepsy. Brain 135:2358–2374
van Vliet EA, Dedeurwaerdere S, Cole AJ et al (2016) WONOEP appraisal: imaging biomarkers in epilepsy. Epilepsia 58:315–330
Ashwal S, Holshouser B, Tong K et al (2004) Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res 56:630–638
Brooks WM, Stidley CA, Petropoulos H et al (2000) Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma 17:629–640
Garnett MR, Blamire AM, Corkill RG et al (2000) Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain 123(Pt 10):2046–2054
Broer S, Loscher W (2015) Novel combinations of phenotypic biomarkers predict development of epilepsy in the lithium-pilocarpine model of temporal lobe epilepsy in rats. Epilepsy Behav 53:98–107
Hitiris N, Mohanraj R, Norrie J et al (2007) Predictors of pharmacoresistant epilepsy. Epilepsy Res 75:192–196
Gastens AM, Brandt C, Bankstahl JP, Loscher W (2008) Predictors of pharmacoresistant epilepsy: pharmacoresistant rats differ from pharmacoresponsive rats in behavioral and cognitive abnormalities associated with experimentally induced epilepsy. Epilepsia 49:1759–1776
Kanner AM, Byrne R, Chicharro A et al (2009) A lifetime psychiatric history predicts a worse seizure outcome following temporal lobectomy. Neurology 72:793–799
de Araujo Filho GM, Gomes FL, Mazetto L et al (2012) Major depressive disorder as a predictor of a worse seizure outcome one year after surgery in patients with temporal lobe epilepsy and mesial temporal sclerosis. Seizure 21:619–623
Cleary RA, Thompson PJ, Thom M, Foong J (2013) Postictal psychosis in temporal lobe epilepsy: risk factors and postsurgical outcome? Epilepsy Res 106:264–272
Gerlai R, Wojtowicz JM, Marks A, Roder J (1995) Overexpression of a calcium-binding protein, S100 beta, in astrocytes alters synaptic plasticity and impairs spatial learning in transgenic mice. Learn Mem 2:26–39
Nishiyama H, Knopfel T, Endo S, Itohara S (2002) Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci USA 99:4037–4042
Huttunen HJ, Kuja-Panula J, Sorci G et al (2000) Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275:40096–40105
Iori V, Maroso M, Rizzi M et al (2013) Receptor for advanced glycation endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis 58:102–114
Mazarati A, Maroso M, Iori V et al (2011) High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp Neurol 232:143–148
Todd KJ, Serrano A, Lacaille JC, Robitaille R (2006) Glial cells in synaptic plasticity. J Physiol Paris 99:75–83
Ota Y, Zanetti AT, Hallock RM (2013) The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast 2013:185463
Ben Achour S, Pascual O (2010) Glia: the many ways to modulate synaptic plasticity. Neurochem Int 57:440–445
Blank T, Prinz M (2013) Microglia as modulators of cognition and neuropsychiatric disorders. Glia 61:62–70
Morris GP, Clark IA, Zinn R, Vissel B (2013) Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem 105:40–53
Donato R, Sorci G, Riuzzi F et al (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793:1008–1022
Morquette P, Verdier D, Kadala A et al (2015) An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat Neurosci 18:844–854
Sakatani S, Seto-Ohshima A, Shinohara Y et al (2008) Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gamma oscillations in vivo. J Neurosci 28:10928–10936
Worrell GA, Parish L, Cranstoun SD et al (2004) High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 127:1496–1506
Koh SXT, Lee JKW (2014) S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med Auckl NZ 44:369–385
Weissberg I, Wood L, Kamintsky L et al (2015) Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis 78:115–125
Friedman A, Heinemann U (2012) Role of Blood-Brain Barrier Dysfunction in Epileptogenesis [Internet]. Jaspers Basic Mech. Epilepsies 4th edition
Friedman A, Kaufer D, Heinemann U (2009) Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res 85:142–149
Maroso M, Balosso S, Ravizza T et al (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–419
Zurolo E, Iyer A, Maroso M et al (2011) Activation of TLR, RAGE and HMGB1 signaling in malformations of cortical development. Brain 134:1015–1032
Walker LE, Frigerio F, Ravizza T et al (2017) Molecular isoforms of HMGB1 are novel mechanistic biomarkers for epilepsy. J Clin Invest (in press)
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Yang H, Antoine DJ, Andersson U, Tracey KJ (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93:865–873
Lamkanfi M, Sarkar A, Vande Walle L et al (2010) Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 185:4385–4392
Yang H, Lundback P, Ottosson L et al (2012) Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 18:250–259
Balosso S, Liu J, Bianchi ME, Vezzani A (2014) Disulfide-containing High Mobility Group Box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal 21:1726–1740
Pauletti A, Terrone G, Shekh-Ahmad T et al (2017) Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain (in press)
Mondello S, Shear DA, Bramlett HM et al (2016) Insight into pre-clinical models of traumatic brain injury using circulating brain damage biomarkers: operation brain trauma therapy. J Neurotrauma 33:595–605
Bronisz E, Kurkowska-Jastrzębska I (2016) Matrix metalloproteinase 9 in epilepsy: the role of neuroinflammation in seizure development. Mediators Inflamm 2016:7369020
Zucker S, Doshi K, Cao J (2004) Measurement of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMP) in blood and urine: potential clinical applications. Adv Clin Chem 38:37–85
Snitker S, Xie K, Ryan KA et al (2013) Correlation of circulating MMP-9 with white blood cell count in humans: effect of smoking. PloS ONE 8:e66277
Wagner S, Breyholz H-J, Faust A et al (2006) Molecular imaging of matrix metalloproteinases in vivo using small molecule inhibitors for SPECT and PET. Curr Med Chem 13:2819–2838
Boison D, Sandau US, Ruskin DN et al (2013) Homeostatic control of brain function—new approaches to understand epileptogenesis. Front Cell Neurosci 7:109
Acknowledgements
We acknowledge the grant support by the European Union’s Seventh Framework Programme (FP7/2007–2013) under Grant Agreement No. 602102 (EPITARGET), and Citizen United for Research in Epilepsy (CURE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vezzani, A., Pascente, R. & Ravizza, T. Biomarkers of Epileptogenesis: The Focus on Glia and Cognitive Dysfunctions. Neurochem Res 42, 2089–2098 (2017). https://doi.org/10.1007/s11064-017-2271-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2271-3