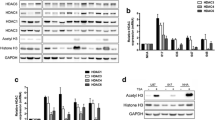
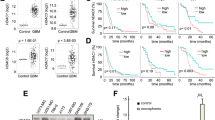
Abstract
Glioblastoma (GBM) is the most frequent and aggressive tumour in the central nervous system. Many studies have demonstrated that upregulation of the NF-κB onco-pathway is accompanied by the acquisition of Temozolomide (TMZ) resistance in GBM cells. Here, we show that RGFP109, a selective histone deacetylase (HDAC1 and HDAC3) inhibitor, overcomes TMZ resistance and downregulates the expression of NF-κB-regulated pro-survival genes in a TMZ-resistant (TR) GBM cell line. RGFP109 did not alter the phosphorylation levels of NF-κB/p65 or inhibitory κBα (IκBα). Immunofluorescence microscopy showed that RGFP109 does not block the nuclear translocation of NF-κB/p65. However, co-immunoprecipitation assays revealed that RGFP109 induces the hyperacetylation of NF-κB/p65 and histones, and blocks interactions between NF-κB/p65 and its coactivators, p300 and p300/CBP-associated factor (PCAF). These results indicate that RGFP109-mediated post-translational nuclear acetylation may be involved in the regulation of NF-κB. Electrophoretic mobility shift assays revealed that RGFP109 reduces NF-κB/p65 binding to κB-DNA and decreased the transcriptional level of κB-mediated genes, suggesting that RGFP109-induced hyperacetylation leads to attenuated transcription of the κB gene. In addition, RGFP109 elevates the expression of inhibitor of growth 4 (ING4), which is typically downregulated in GBM cells. Importantly, we found that RGFP109 enhances ING4 recognition and binding to NF-κB/p65, which may be positively correlated with reduced interactions between NF-κB/p65 and p300/PCAF, thereby effecting transcription of the κB gene. Finally, we show that knockdown of ING4 with plasmids containing pcDNA3.1-ING4 shRNA abolished the effect of RGFP109. Therefore, ING4 may act as a corepressor and facilitate RGFP109-triggered suppression of the NF-κB pathway. Taken together, our data show that RGFP109, an HDAC inhibitor, in combination with TMZ may be a therapeutic candidate for patients with temozolomide-resistant GBM.





Similar content being viewed by others
References
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. The Lancet 359:1011–1018
Kokkinakis DM, Bocangel DB, Schold SC, Moschel RC, Pegg AE (2001) Thresholds of O6-alkylguanine-DNA alkyltransferase which confer significant resistance of human glial tumor xenografts to treatment with 1,3-bis(2-chloroethyl)-1-nitrosourea or temozolomide. Clin Cancer Res 7:421–428
Bredel M, Bredel C, Juric D, Duran GE, Yu RX, Harsh GR, Vogel H, Recht LD, Scheck AC, Sikic BI (2006) Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-κB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol 24:274–287
Atkinson GP, Nozell SE, Benveniste ET (2010) NF-κB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother 10:575–586
Nozell S, Laver T, Moseley D, Nowoslawski L, De Vos M, Atkinson GP, Harrison K, Nabors LB, Benveniste EN (2008) The ING4 tumor suppressor attenuates NF-κB activity at the promoters of target genes. Mol Cell Biol 28:6632–6645
Kapoor GS, Zhan Y, Johnson GR, O’Rourke DM (2004) Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-κB activation through Gab1 in glioblastoma cells. Mol Cell Biol 24:823–836
Greene WC, Chen LF (2004) Regulation of NF-κB action by reversible acetylation. Novartis Found Symp 259:208–217 (discussion 218–225)
Vermeulen L, De Wilde G, Van Damme P, Berghe WV, Haegeman G (2003) Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22:1313–1324
Perkins ND, Gilmore TD (2006) Good cop, bad cop: the different faces of NF-κB. Cell Death Differ 13:759–772
Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T (1999) Transcriptional activation by NF-κB requires multiple coactivators. Mol Cell Biol 19:6367–6378
Zhong H, Voll RE, Ghosh S (1998) Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1:661–671
Chen L, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653–1657
Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M (2003) Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. J Biol Chem 278:2758–2766
Saito A, Yamashita T, Mariko Y, Nosaka Y, Tsuchiya K, Ando T, Suzuki T, Tsuruo T, Nakanishi O (1999) A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci USA 96:4592–4597
Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA (2013) HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci USA 110:2647–2652
Johnston TH, Huot P, Damude S, Fox SH, Jones SW, Rusche JR, Brotchie JM (2013) RGFP109, a histone deacetylase inhibitor attenuates l-DOPA-induced dyskinesia in the MPTP-lesioned marmoset: a proof-of-concept study. Parkinsonism Relat Disord 19:260–264
Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK (2004) The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature 428:328–332
Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang X, Zhang X (2007) Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol 85:263–270
Perkins ND (2006) Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene 25:6717–6730
Wolffe AP (1996) Histone deacetylase: a regulator of transcription. Science 272:371–372
Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119:555–566
Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R (2012) The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 151:1185–1199
Hu E, Dul E, Sung CM, Chen Z, Kirkpatrick R, Zhang GF, Johanson K, Liu R, Lago A, Hofmann G, Macarron R, de los Frailes M, Perez P, Krawiec J, Winkler J, Jaye M (2003) Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Therap 307:720–728
Werner SL, Barken D, Hoffmann A (2005) Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science 309:1857–1861
Furia B, Deng L, Wu K, Baylor S, Kehn K, Li H, Donnelly R, Coleman T, Kashanchi F (2002) Enhancement of nuclear factor-κB acetylation by coactivator p300 and HIV-1 Tat proteins. J Biol Chem 277:4973–4980
Quivy V, Van Lint C (2004) Regulation at multiple levels of NF-κB-mediated transactivation by protein acetylation. Biochem Pharmacol 68:1221–1229
Hoberg JE, Yeung F, Mayo MW (2004) SMRT derepression by the IκB kinase alpha: a prerequisite to NF-κB transcription and survival. Mol Cell 16:245–255
Roth SY, Denu JM, Allis CD (2001) Histone acetyltransferases. Annu Rev Biochem 70:81–120
Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G (1999) The nuclear factor-κB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem 274:32091–32098
Kaina B, Christmann M, Naumann S, Roos WP (2007) MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Rep 6:1079–1099
Suka N, Carmen AA, Rundlett SE, Grunstein M (1998) The regulation of gene activity by histones and the histone deacetylase RPD3. Cold Spring Harb Symp Quant Biol 63:391–399
Camphausen K, Burgan W, Cerra M, Oswald KA, Trepel JB, Lee MJ, Tofilon PJ (2004) Enhanced radiation-induced cell killing and prolongation of \(\gamma\)H2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res 64:316–321
Eyupoglu IY, Hahnen E, Trankle C, Savaskan NE, Siebzehnrubl FA, Buslei R, Lemke D, Wick W, Fahlbusch R, Blumcke I (2006) Experimental therapy of malignant gliomas using the inhibitor of histone deacetylase MS-275. Mol Cancer Ther 5:1248–1255
Palacios A, Garcia P, Padro D, Lopez-Hernandez E, Martin I, Blanco FJ (2006) Solution structure and NMR characterization of the binding to methylated histone tails of the plant homeodomain finger of the tumour suppressor ING4. FEBS Lett 580:6903–6908
Pena PV, Davrazou F, Shi X, Walter KL, Verkhusha VV, Gozani O, Zhao R, Kutateladze TG (2006) Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442:100–103
Gong W, Suzuki K, Russell M, Riabowol K (2005) Function of the ING family of PHD proteins in cancer. Int J Biochem Cell Biol 37:1054–1065
Campos EI, Chin MY, Kuo WH, Li G (2004) Biological functions of the ING family tumor suppressors. Cell Mol Life Sci 61:2597–2613
Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Pena P, Lan F, Kaadige MR, Lacoste N, Cayrou C, Davrazou F, Saha A, Cairns BR, Ayer DE, Kutateladze TG, Shi Y, Cote J, Chua KF, Gozani O (2006) ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442:96–99
Doyon Y, Cayrou C, Ullah M, Landry AJ, Cote V, Selleck W, Lane WS, Tan S, Yang XJ, Cote J (2006) ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell 21:51–64
Shiseki M, Nagashima M, Pedeux RM, Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y, Appella E, Yokota J, Harris CC (2003) p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res 63:2373–2378
Zhang X, Wang KS, Wang ZQ, Xu LS, Wang QW, Chen F, Wei DZ, Han ZG (2005) Nuclear localization signal of ING4 plays a key role in its binding to p53. Biochem Biophys Res Commun 331:1032–1038
Gunduz M, Nagatsuka H, Demircan K, Gunduz E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K, Beder L, Hirohata S, Ninomiya Y, Nishizaki K, Shimizu K, Nagai N (2005) Frequent deletion and down-regulation of ING4, a candidate tumor suppressor gene at 12p13, in head and neck squamous cell carcinomas. Gene 356:109–117
Author information
Authors and Affiliations
Corresponding author
Additional information
Zong-yang Li and Qing-zhong Li have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Zy., Li, Qz., Chen, L. et al. Histone Deacetylase Inhibitor RGFP109 Overcomes Temozolomide Resistance by Blocking NF-κB-Dependent Transcription in Glioblastoma Cell Lines. Neurochem Res 41, 3192–3205 (2016). https://doi.org/10.1007/s11064-016-2043-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2043-5