Abstract
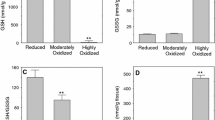
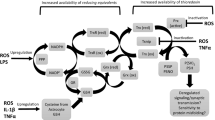
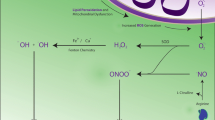
Oxidative stress is a long-hypothesized cause of diverse neurological and psychiatric disorders but the pathways by which physiological redox perturbations may detour healthy brain development and aging are unknown. We reported recently (Foley et al., Neurochem Res 39:2030–2039, 2014) that two-electron oxidations, to disulfides, of protein vicinal thiols can vary markedly in association with more modest oxidations of the glutathione redox couple in brains from healthy adolescent rats whereas levels of protein S-glutathionylation were low and unchanged. Here, we demonstrate that the selective oxidations of protein vicinal thiols, occurring only in the more oxidized brains under study, were linked specifically to a peroxide stress as evidenced by increased oxidations, to disulfides, of the presumed catalytic vicinal thiols of peroxiredoxins 1 and 2. Moreover, we identify the catalytic subunit(s) of Na+, K+-ATPase, tubulins, glyceraldehyde-3-phosphate dehydrogenase, and protein phosphatase 1, all of which can modulate glutamate neurotransmission and the vulnerability of neurons to excitotoxicity, as non-peroxidase proteins exhibiting prominent oxidations of vicinal thiols. The two-electron pathway, demonstrated here, linking physiological redox perturbations in otherwise healthy brains to protein determinants of excitotoxicity, suggests an alternative to free radical pathways by which oxidative stress may impact brain development and aging.



Similar content being viewed by others
References
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Rad Biol Med 23:134–147
Emiliani FE, Sedlak TW, Sawa A (2014) Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr Opin Psychiatry 27:185–190
Jones DP (2008) Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295:C849–C868
Randall LM, Ferrer-Sueta G, Denicola A (2013) Peroxiredoxins as preferential targets in H2O2-induced signaling. Methods Enzymol 527:41–63
Du Y, Zhang H, Lu J, Holmgren A (2012) Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J Biol Chem 287:38210–38219
Folbergrova J, Rehncrona S, Siesjo BK (1979) Oxidized and reduced glutathione in the rat brain under normoxic and hypoxic conditions. J Neurochem 32:1621–1627
Rebrin I, Sohal RS (2004) Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol 39:1513–1519
Rebrin I, Forster MJ, Sohal RS (2007) Effects of age and caloric restriction on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res 1127:10–18
Foley TD, Cantarella KM, Gillespie PF, Stredny ES (2014) Protein vicinal thiol oxidations in the healthy brain: not so radical links between physiological oxidative stress and neural cell activities. Neurochem Res 39:2030–2039
Adams E, Jeter D, Cordes AW, Kolis JW (1990) Chemistry of organometalloid complexes with potential antidotes: structure of an organoarsenic(III) dithiolate ring. Inorg Chem 29:1500–1503
Foley TD, Stredny CM, Coppa TM, Gubbiotti MA (2010) An improved phenylarsine oxide-affinity method identifies triose phosphate isomerase as a candidate redox receptor protein. Neurochem Res 35:306–314
Foley TD, Melideo SL, Healey AE, Lucas EJ, Koval JA (2011) Phenylarsine oxide binding reveals redox-active and potential regulatory vicinal thiols on the catalytic subunit of protein phosphatase 2A. Neurochem Res 236:232–240
Foley TD, Clark AR, Stredny ES, Wierbowski BM (2012) SNAP-25 contains non-acylated thiol pairs that can form intrachain disulfide bonds: possible sites for redox modulation of neurotransmission. Cell Mol Neurobiol 32:201–208
Gemba T, Oshima T, Ninomiya M (1994) Glutamate efflux via the reversal of the sodium-dependent glutamate transporter caused by glycolytic inhibition in rat cultured astrocytes. Neuroscience 63:789–795
Brines ML, Dare AO, de Lanerolle NC (1995) The cardiac glycoside ouabain potentiates excitotoxic injury of adult neurons in rat hippocampus. Neurosci Lett 191:145–148
Matthews RT, Ferrante RJ, Jenkins BG, Browne SE, Goetz K, Berger S, Chen IY, Beal MF (1997) Iodoacetate produces striatal excitotoxic lesions. J Neurochem 69:285–289
Massieu L, Gómez-Román N, Montiel T (2000) In vivo potentiation of glutamate mediated neuronal damage after chronic administration of the glycolysis inhibitor iodoacetate. Exp Neurol 165:257–267
Sistiaga A, Sánchez-Prieto J (2000) Protein phosphatase 1 and 2A inhibitors prolong the switch in the control of glutamate release by group I metabotropic glutamate receptors: characterization of the inhibitory pathway. J Neurochem 75:1566–1574
Veldhuis WB, van der Stelt M, Delmas F, Gillet B, Veldink GA, Vliegenthart JF, Nicolay K, Bär PR (2003) In vivo excitotoxicity induced by ouabain, a Na+/K+-ATPase inhibitor. J Cereb Blood Flow Metab 23:62–74
Yuen EY, Jiang Q, Feng J, Yan Z (2005) Microtubule regulation of N-methyl-D-aspartate receptor channels in neurons. J Biol Chem 280:29420–29427
Farinelli M, Heitz FD, Grewe BF, Tyagarajan SK, Helmchen F, Mansuy IM (2012) Selective regulation of NR2B by protein phosphatase-1 for the control of the NMDA receptor in neuroprotection. PLoS One 7:e34047
Hwang NR, Yim SH, Kim YM, Jeong J, Song EJ, Lee Y, Lee JH, Choi S, Lee KJ (2009) Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem J 423:253–264
Peralta D, Bronowska AK, Morgan B, Dóka É, Van Laer K, Nagy P, Gräter F, Dick TP (2015) A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol 11:156–163
Ralser M, Wamelink MM, Kowald A, Gerisch B, Heeren G, Struys EA, Klipp E, Jakobs C, Breitenbach M, Lehrach H, Krobitsch S (2007) Dynamic rerouting of carbohydrate flux is key to counteracting oxidative stress. J Biol 6:10
Chaudhuri AR, Khan IA, Ludueña RF (2001) Detection of disulfide bonds in bovine brain tubulin and their role in protein folding and microtubule assembly in vitro: a novel disulfide detection approach. Biochemistry 40:8834–8841
Tyson PA, Steinberg M, Wallick ET, Kirley TL (1989) Identification of the 5-iodoacetamidofluorescein reporter site on the Na, K-ATPase. J Biol Chem 264:726–734
Fetrow JS, Siew N, Skolnick J (1999) Structure-based functional motif identifies a potential disulfide oxidoreductase active site in the serine/threonine protein phosphatase-1 subfamily. FASEB J 1999:1866–1874
Netto LE, Antunes F (2016) The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol Cells 39:65–71
Yan H, Lou MF, Fernando MR, Harding JJ (2006) Thioredoxin, thioredoxin reductase, and alpha-crystallin revive inactivated glyceraldehyde 3-phosphate dehydrogenase in human aged and cataract lens extracts. Mol Vis 12:1153–1159
Khan IA, Ludueña RF (1991) Possible regulation of the in vitro assembly of bovine brain tubulin by the bovine thioredoxin system. Biochim Biophys Acta 1076:289–297
Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S (2010) Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr 47:224–232
Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, Han PL (2012) NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci 32:9690–9699
Caccamo A, Medina DX, Oddo S (2013) Glucocorticoids exacerbate cognitive deficits in TDP-25 transgenic mice via a glutathione-mediated mechanism: implications for aging, stress and TDP-43 proteinopathies. J Neurosci 33:906–913
Tuli JS, Smith JA, Morton DB (1995) Stress measurements in mice after transportation. Lab Anim 29:132–138
Hackett MJ, Britz CJ, Paterson PG, Nichol H, Pickering IJ, George GN (2015) In situ biospectroscopic investigation of rapid ischemic and postmortem induced biochemical alterations in the rat brain. ACS Chem Neurosci 6:226–238
Sapolsky RM (1986) Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J Neurosci 6:2240–2244
Sapolsky RM (1986) Glucocorticoid toxicity in the hippocampus. Temporal aspects of synergy with kainic acid. Neuroendocrinology 43:440–444
Acknowledgments
This work was funded by Internal Research and Faculty Resource Allocation Plan Grants from the University of Scranton.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal Use
Experiments involving animals were approved by the University of Scranton Institutional Animal Care and Use Committee in accordance with ethical standards for the use of animals in scientific research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Foley, T.D., Katchur, K.M. & Gillespie, P.F. Disulfide Stress Targets Modulators of Excitotoxicity in Otherwise Healthy Brains. Neurochem Res 41, 2763–2770 (2016). https://doi.org/10.1007/s11064-016-1991-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1991-0