Abstract
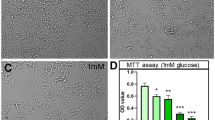
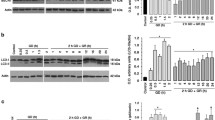
Glucose is the major energy substrate in brain, however, during ketogenesis induced by starvation or prolonged hypoglycemia, the ketone bodies (KB), acetoacetate and β-hydroxybutyrate (BHB) can substitute for glucose. KB improve neuronal survival in diverse injury models, but the mechanisms by which KB prevent neuronal damage are still not well understood. In the present study we have investigated whether protection by the D isomer of BHB (D-BHB) against neuronal death induced by glucose deprivation (GD), is related to autophagy. Autophagy is a lysosomal-dependent degradation process activated during nutritional stress, which leads to the digestion of damaged proteins and organelles providing energy for cell survival. Results show that autophagy is activated in cortical cultured neurons during GD, as indicated by the increase in the levels of the lipidated form of the microtubule associated protein light chain 3 (LC3-II), and the number of autophagic vesicles. At early phases of glucose reintroduction (GR), the levels of p62 declined suggesting that the degradation of the autophagolysosomal content takes place at this time. In cultures exposed to GD and GR in the presence of D-BHB, the levels of LC3-II and p62 rapidly declined and remained low during GR, suggesting that the KB stimulates the autophagic flux preventing autophagosome accumulation and improving neuronal survival.






Similar content being viewed by others
Abbreviations
- GD:
-
Glucose deprivation
- GR:
-
Glucose reperfusion
- LC3:
-
Microtubule associated protein light chain 3
- LC3-II:
-
Lipidated form of the microtubule associated protein light chain 3
- BHB:
-
β-Hydoxybutyrate
- D-BHB:
-
D isomer of β-hydoxybutyrate
- 3-MA:
-
3-Methyl adenine
- KB:
-
Ketone bodies
References
Robinson AM, Williamsom DH (1980) Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60:143–187
Hawkins RA, Williamson DH, Krebs HA (1971) Ketone body utilization by adult and suckling rat brain in vivo. Biochem J 122:13–18
Nehlig A, Pereira de Vasconcelos A (1993) Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol 40:163–221
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr (1967) Brain metabolism during fasting. J Clin Invest 46:1589–1595
Pan JW, de Graaf R, Petersen KF, Shulman G, Herrington HP, Rothman DL (2002) [2,4-13C2]-beta-hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 22:890–898
Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I (2001) Ketogenic diet, amino acid metabolism and seizure control. J Neurosci Res 66:931–940
Masuda R, Monahan JW, Kashiwaya Y (2005) D-beta-hydroxybutyrate is neuroprotective against hypoxia in serum-free hippocampal primary cultures. J Neurosci Res 80:501–509
Suzuki M, Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S et al (2002) β-Hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn J Pharmacol 89:36–43
Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S et al (2008) Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab 28:1907–1916
Tai KK, Nguyen N, Pham L, Truong DD (2008) Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J Neural Transm 115:1011–1017
Massieu L, Haces ML, Montiel T, Hernández-Fonseca K (2003) Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience 120:365–378
Noh HS, Hah YS, Nilufar R, Han J, Bong JH, Kang SS et al (2006) Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J Neurosci Res 83:702–709
Yamada KA, Rensing N, Thio LL (2005) Ketogenic diet reduces hypoglycemia-induced neuronal death in young rats. Neurosci Lett 385:210–214
Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L (2008) Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol 211:85–96
Julio-Amilpas A, Montiel T, Soto-Tinoco E, Gerónimo-Olvera C, Massieu L (2015) Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab 35:851–860
Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM (2007) Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 145:256–264
Zhang J, Cao Q, Li S, Lu X, Zhao Y, Guan JS et al (2013) 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 34:7552–7562
Mizushima N, Levine B, Cuervo AM, Klionsky D (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Tanida I (2011) Autophagy basics. Microbiol Immunol 55:1–11
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293
Alirezaei M, Kembal CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB (2010) Short-term fasting induces profound neuronal autophagy. Autophagy 6:702–710
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programed cell death. Cell Death Diff 12:1528–1534
Clarke PGH, Puyal J (2012) Autophagic cell death exists. Autophagy 8:867–869
Sou Y-S, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T et al (2008) The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell 19:4762–4775
Kulbe JR, Levy JMM, Coultrap SJ, Thorburn A, Baye KU (2014) Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res 1542:12–19
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM (2014) Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 10:2208–2222
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884
Carloni S, Buonocore G, Balduini W (2008) Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 32:329–339
Bukcley KM, Hess DL, Sazonova IY, Periyasamy-Thandavan S, Barrett JR, Kirks R et al (2014) Rapamycin up-regulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL, and embolic MCAO, murine models of stroke. Exp Trans Stroke Med 6:8
Shi R, Weng J, Zhao L, Li X-M, Gao T-M, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosc Ther 18:25–260
Higgins GC, Devenish RJ, Beart PM, Nagley P (2011) Autophagic activity in cortical neurons under acute oxidative stress directly contributes to cell death. Cell Mol Life Sci 68:3725–3740
Jang BG, Choi BY, Kim JH, Kim MJ, Sohn M, Suh SW (2013) Impairment of autophagic flux promotes glucose reperfusion-induced neuro2A cell death after glucose deprivation. PLoS ONE 8:e76466
Yue Z, Zhong Y (2010) From global view to focused examination: understanding cellular function of lipid kinase VPS34-Beclin 1 complex in autophagy. J Mol Cell Biol 2:305–307
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, et al (2012) A novel image-based cytometry method for autophagy detection in living cells. Autophagy 8:1371–1382
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD et al (2008) Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4:762–769
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S et al (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172:454–469
Liu C, Gao Y, Barrett J, Hu B (2010) Autophagy and protein aggregation after brain ischemia. J Neurochem 115:68–78
Balmer D, Emery M, Andreux P, Auwerx J, Ginet V, Puyal J et al (2013) Autophagy defect is associated with low glucose-induced apoptosis in 66 W photoreceptor cells. PLoS ONE 8:e74162
Izumi Y, Benz AM, Katsuki H, Zorumski CF (1997) Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci 17:9448–9457
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Acknowledgments
This study was performed in partial fulfillment of the requirements for the Ph.D. degree in Ciencias Biomédicas of L. Camberos-Luna at the Universidad Nacional Autónoma de México. This work was supported by Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT) grant IN204213 and Consejo Nacional de Ciencia y Tecnología (CONACYT) Grant CB-239607 to LM and CONACYT scholarship to L. Camberos-Luna. Authors thank Augusto César Poot-Hernández for his help in vesicle counting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Special Issue: In Honor of Philip Beart.
Lucy Camberos-Luna and Cristian Gerónimo-Olvera have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Camberos-Luna, L., Gerónimo-Olvera, C., Montiel, T. et al. The Ketone Body, β-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem Res 41, 600–609 (2016). https://doi.org/10.1007/s11064-015-1700-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1700-4