Abstract
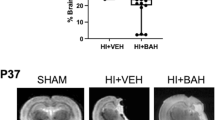
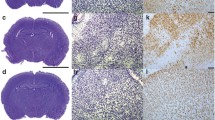
Hypoxia leads to activation of many cellular adaptive processes which are regulated by the transcription factor hypoxia-inducible factor-1 (HIF-1). HIF-1 consists of HIF-1α and HIF-1ß subunits and levels of HIF-1α protein are regulated by HIF prolyl-hydroxylase enzymes (PHD1, 2, 3). The aim of the current study was to investigate the expression of HIF-1α and PHDs at various time points after hypoxia–ischemia (HI), using a neonatal rat model of HI brain injury. Sprague–Dawley rat pups (postnatal day 7) were anaesthetized and underwent right carotid artery occlusion and were then exposed to 6 % oxygen for 2.5 h at 37 °C. HI injured animals demonstrated a significant reduction in the size of the ipsilateral hemisphere, compared to sham controls. Protein analysis using western blotting and enzyme-linked immunosorbent assay showed that 24 h after HI, there was a significant increase in PHD3 protein and an increase of HIF-1α compared to controls. At the 72 h time point, there was a reduction in PHD3 protein, which appeared to relate to cellular loss. There were no changes in PHD1 or PHD2 protein levels after HI when compared to age-matched controls. Further studies are necessary to establish roles for the HIF-1 regulatory enzyme PHD3 in brain injury processes.




Similar content being viewed by others
References
Ferriero DM (2004) Neonatal brain injury. N Engl J Med 351(19):1985–1995
Gonzalez FF, Ferriero DM (2008) Therapeutics for neonatal brain injury. Pharmacol Ther 120(1):43–53
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292(5516):468–472
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294(5545):1337–1340
Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW (2000) Activation of HIF1 alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci USA 97(19):10430–10435
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107(1):43–54
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J (2003) HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22(16):4082–4090
Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH (2006) Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 281(33):23482–23491
D’Angelo G, Duplan E, Boyer N, Vigne P, Frelin C (2003) Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem 278(40):38183–38187
Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, Murphy M, Derr J, Taya Y, Lowe SW, Kastan M, Giaccia A (2001) Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol 21(4):1297–1310
Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS (2003) Hypoxia-inducible factor 1 alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 23(1):359–369
Althaus J, Bernaudin M, Petit E, Toutain J, Touzani O, Rami A (2006) Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int 48(8):687–695
Halterman MW, Federoff HJ (1999) HIF-1 alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol 159(1):65–72
Vangeison G, Carr D, Federoff HJ, Rempe DA (2008) The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci 28(8):1988–1993
Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC (2007) Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27(23):6320–6332
Sheldon RA, Lee CL, Jiang X, Knox RN, Ferriero DM (2014) Hypoxic preconditioning protection is eliminated in HIF-1α knockout mice subjected to neonatal hypoxia-ischemia. Pediatr Res 76(1):46–53
Jones NM, Lee EM, Brown TG, Jarrott B, Beart PM (2006) Hypoxic preconditioning produces differential expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and its regulatory enzyme HIF prolyl hydroxylase 2 in neonatal rat brain. Neurosci Lett 404(1–2):72–77
Rice J, Vannucci R, Brierly J (1981) The influence of immaturity on hypoxic–ischemic brain damage in the rat. Ann Neurol 9:131–141
Chen W, Jadhav V, Tang J, Zhang JH (2008) HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic–ischemic model. Neurobiol Dis 31(3):433–441
Jones NM, Kardashyan L, Callaway JK, Lee EM, Beart PM (2008) Long-term functional and protective actions of preconditioning with hypoxia, cobalt chloride, and desferrioxamine against hypoxic–ischemic injury in neonatal rats. Pediatr Res 63(6):620–624
van den Tweel ER, Kavelaars A, Lombardi MS, Nijboer CH, Groenendaal F, van Bel F, Heijnen CJ (2006) Bilateral molecular changes in a neonatal rat model of unilateral hypoxic–ischemic brain damage. Ped Res 59(3):434–439
Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15(13):2445–2453
Li L, Qu Y, Li J, Xiong Y, Mao M, Mu D (2007) Relationship between HIF-1alpha expression and neuronal apoptosis in neonatal rats with hypoxia–ischemia brain injury. Brain Res 1180:133–139
Henze ATRJ, Diem T, Wenner J, Flamme I, Pouyseggur J, Plate KH, Acker T (2010) Prolyl hydroxylases 2 and 3 act in gliomas as protective negative feedback regulators of hypoxia-inducible factors. Cancer Res 70(1):357–366
Madden SL, Galella EA, Riley D, Bertelsen AH, Beaudry GA (1996) Induction of cell growth regulatory genes by p53. Cancer Res 56(23):5384–5390
Straub JA, Lipscomb EA, Yoshida ES, Freeman RS (2003) Induction of SM-20 in PC12 cells leads to increased cytochrome c levels, accumulation of cytochrome c in the cytosol, and caspase-dependent cell death. J Neurochem 85(2):318–328
Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, Farese RV, Freeman RS, Carter BD, Kaelin WG Jr, Schlisio S (2005) Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell 8(2):155–167
Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC, Shaw G, Parmar S, Schneider M, Sabroe I, Dockrell DH, Milo M, Taylor CT, Johnson RS, Pugh CW, Ratcliffe PJ, Maxwell PH, Carmeliet P, Whyte MK (2011) Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest 121:1053–1063
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279(37):38458–38465
Metzen E, Stiehl DP, Doege K, Marxsen JH, Hellwig-Burgel T, Jelkmann W (2005) Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene: identification of a functional hypoxia-responsive element. Biochem J 387(Pt 3):711–717
Serra-Perez AAMP, Nunez-O’Mara A, Berra E, Garcia-Villoria J, Ribes A, Santalucia T (2010) Extended ischemia prevents HIF1alpha degradation at reoxygenation by impairing prolyl-hydroxylation: role of Krebs cycle metabolites. J Biol Chem 285:18217–18224
Jones NM, Bergeron M (2001) Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab 21 (9)(42):1105–1114
Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR (2002) Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem 277(42):39728–39738
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Dr. Philip Beart.
Rights and permissions
About this article
Cite this article
Chu, H.X., Jones, N.M. Changes in Hypoxia-Inducible Factor-1 (HIF-1) and Regulatory Prolyl Hydroxylase (PHD) Enzymes Following Hypoxic–Ischemic Injury in the Neonatal Rat. Neurochem Res 41, 515–522 (2016). https://doi.org/10.1007/s11064-015-1641-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1641-y