Abstract
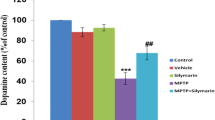
In neurodegenerative disorders such as Parkinson’s disease (PD), autophagy is implicated in the process of dopaminergic neuron cell death. The α-synuclein protein is a major component of Lewy bodies and Lewy neurites, and mutations in α-synuclein have been implicated in the etiology of familial PD. The current work investigates the mechanisms underlying the therapeutic effects of the autophagy-stimulating antibiotic rapamycin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. Male C57BL/6 mice were treated with intravenous rapamycin or saline control for 7 days following MPTP administration. Immunohistochemistry and western blotting were used to detect alterations in the expression of PD biomarkers, including tyrosine hydroxylase (TH), and the level of autophagy was evaluated by the detection of both microtubule-associated protein light chain 3 (LC3) and α-Synuclein cleavage. In addition, levels of monoamine neurotransmitters were measured in the striatum using high performance liquid chromatography (HPLC). Immunohistochemistry using antibodies against TH indicated that the number of dopaminergic neurons in the substantia nigra following MPTP treatment was significantly higher in rapamycin-treated mice compared with saline-treated controls (p < 0.01). Levels of TH expression in the striatum were similar between the groups. α-synuclein Immunoreactivity was significantly decreased in rapamycin-treated mice compared with controls (p < 0.01). Immunoreactivity for LC3, however, was significantly higher in the rapamycin-treated animals than controls (p < 0.01). The concentrations of both striatal dopamine, and the dopamine metabolite DOPAC, were significantly decreased in both MPTP-treated groups compared with untreated controls. The loss of DOPAC was less severe in rapamycin-treated mice compared with saline-treated mice (p < 0.01) following MPTP treatment. These results demonstrate that treatment with rapamycin is able to prevent the loss of TH-positive neurons and to ameliorate the loss of DOPAC following MPTP treatment, likely via activation of autophagy/lysosome pathways. Thus, further investigation into the effectiveness of rapamycin administration in the treatment of PD is warranted.






Similar content being viewed by others
References
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535
Giasson BI, Duda JE, Murray IV et al (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290(5493):985–989
Spillantini MG, Schmidt ML, Lee VM et al (1997) Alpha-synuclein in lewy bodies. Nature 388(6645):839–840
Polymeropoulos MH (1998) Autosomal dominant Parkinson’s disease and alpha-synuclein. Ann Neurol 44(3 Suppl 1):S63–S64
Liu KY, Liu CF, Qian JJ (2008) Autophagic pathway and probable mechanism in degradation of mutant α-synuclein in PC12 cells. Chin J Neurol 41(1):51–56
Anglade P, Vyas S, Javoy-Agid F et al (1997) Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 12(1):25–31
Kruger R, Kuhn W, Muller T et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108
Yang YPLK, Qian JJ et al (2007) The abnormal aggregation of alpha-synuclein induces autophagic programmed cell death in PC12 cells. Eur J Neurol 14(Suppl):341–343
Kamada Y, Funakoshi T, Shintani T et al (2000) Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol 150(6):1507–1513
Yu L, Alva A, Su H et al (2004) Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304(5676):1500–1502
Ohsawa Y, Isahara K, Kanamori S et al (1998) An ultrastructural and immunohistochemical study of PC12 cells during apoptosis induced by serum deprivation with special reference to autophagy and lysosomal cathepsins. Arch Histol Cytol 61(5):395–403
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477
Shimizu S, Kanaseki T, Mizushima N et al (2004) Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 6(12):1221–1228
Kanzawa T, Zhang L, Xiao L et al (2005) Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene 24(6):980–991
Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443(7113):780–786
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Chan L, Gaston R, Hariharan S (2001) Evolution of immunosuppression and continued importance of acute rejection in renal transplantation. Am J Kidney Dis 38(6 Suppl 6):S2–S9
Moore DJ, West AB, Dawson VL et al (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87
Fujiwara H, Hasegawa M, Dohmae N et al (2002) Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4(2):160–164
Rieker C, Engblom D, Kreiner G et al (2011) Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci 31(2):453–460
Hsu LJ, Sagara Y, Arroyo A et al (2000) Alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157(2):401–410
Petrucelli L, Dawson TM (2004) Mechanism of neurodegenerative disease: role of the ubiquitin proteasome system. Ann Med 36(4):315–320
Song DD, Shults CW, Sisk A et al (2004) Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol 186(2):158–172
Webb JL, Ravikumar B, Atkins J et al (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278(27):25009–25013
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Mizushima N, Sugita H, Yoshimori T et al (1998) A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem 273(51):33889–33892
Abeliovich H, Dunn WA Jr, Kim J et al (2000) Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. J Cell Biol 151(5):1025–1034
Sarkar S, Davies JE, Huang Z et al (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282(8):5641–5652
Wellington CL, Singaraja R, Ellerby L et al (2000) Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J Biol Chem 275(26):19831–19838
Malagelada C, Jin ZH, Greene LA (2008) RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci 28(53):14363–14371
Malagelada C, Jin ZH, Jackson-Lewis V et al (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci 30(3):1166–1175
Ravikumar B, Berger Z, Vacher C et al (2006) Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet 15(7):1209–1216
Klionsky DJ, Ohsumi Y (1999) Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol 15:1–32
Acknowledgments
This study was supported by National Natural Science Foundation of China (No.30970869 and No.31171014), Board of Health of Shanghai, China (No.2008086) and by grants from the project of Shanghai Key Laboratory of Diabetes Mellitus (08DZ2230200), meanwhile, this project was also granted by Key Young Project of Fudan University (09L-37) and the Science and Technology Commission of Shanghai Municipality(09DZ1950400).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, K., Shi, N., Sun, Y. et al. Therapeutic Effects of Rapamycin on MPTP-Induced Parkinsonism in Mice. Neurochem Res 38, 201–207 (2013). https://doi.org/10.1007/s11064-012-0909-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0909-8