Abstract
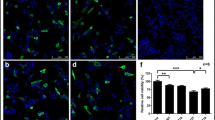
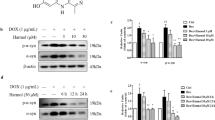
Accumulation of α-synuclein (α-Syn) is a common pathology for both familiar and sporadic Parkinson’s disease (PD), enhancing its clearance might be a promising strategy for treating PD. To assess the potential of trehalose in this regard, we investigated its effect on the PC12 cells overexpressing wild type (WT) or A53T mutant α-Syn and the implicated pathway it might mediated. We observed that trehalose promoted the clearance of A53T α-Syn but not WT α-Syn in PC12 cells, and confirmed the increased LC3 and Lysotracker RED positive autolysosomes by using lysotracker and LC3 staining, the enhanced expression of LC3-II in Western blot, and more autophagosomes under Transmission Electron Microscope in a dose dependent manner after the trehalose treatment. The activation of autophagy can be alleviated by applying macroautophagy inhibitor 3-methyladenine (3-MA). In addition, degradation of A53T and WT α-Syn was blocked after Ubiquitin Proteasome System (UPS) inhibitor (MG132) was applied in those PC12 cells overexpressing A53T or WT α-Syn, suggesting that A53T α-Syn could be degraded by both UPS and macroautophagy. But the effect of trehalose on A53T α-Syn is mainly mediated through the macroautophagy pathway, which is not a dominant way for WT α-Syn clearance. Further in vivo research will be needed to verify the effectiveness of trehalose in treating PD.





Similar content being viewed by others
Abbreviations
- α-Syn:
-
α-synuclein
- PD:
-
Parkinson’s disease
- WT:
-
Wild type
- 3-MA:
-
3-methyladenine
- UPS:
-
Ubiquitin Proteasome System
- ALS:
-
Autophagy Lysosome System
- TEM:
-
Transmission electron microscope
- DMSO:
-
Dimethyl sulfoxide
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-dipheyltetrazolium Bromide assay
References
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840
Korolchuk VI, Menzies FM, Rubinsztein DC (2009) A novel link between autophagy and the ubiquitin-proteasome system. Autophagy 5:862–863
Lee FK, Wong AK, Lee YW, Wan OW, Chan HY, Chung KK (2009) The role of ubiquitin linkages on alpha-synuclein induced-toxicity in a Drosophila model of Parkinson’s disease. J Neurochem 110:208–219
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978
McNaught KS, Jenner P (2001) Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci Lett 297:191–194
Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R (2004) Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med 351:1972–1977
Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA (2001) Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci 21:9549–9560
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013
Rott R, Szargel R, Haskin J et al (2008) Monoubiquitylation of alpha-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J Biol Chem 283:3316–3328
Chen Q, Haddad GG (2004) Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol 207:3125–3129
Ohtake S, Wang YJ (2011) Trehalose: current use and future applications. J Pharm Sci 100:2020–2053
Welch WJ, Brown CR (1996) Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1:109–115
Yu WB, Jiang T, Lan DM et al (2012) Trehalose inhibits fibrillation of A53T mutant alpha-synuclein and disaggregates existing fibrils. Arch Biochem Biophys 523:144–150
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282:5641–5652
Casarejos MJ, Solano RM, Gomez A, Perucho J, de Yebenes JG, Mena MA (2011) The accumulation of neurotoxic proteins, induced by proteasome inhibition, is reverted by trehalose, an enhancer of autophagy, in human neuroblastoma cells. Neurochem Int 58:512–520
Tanaka M, Machida Y, Niu S et al (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10:148–154
Arora A, Ha C, Park CB (2004) Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett 564:121–125
Liu R, Barkhordarian H, Emadi S, Park CB, Sierks MR (2005) Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol Dis 20:74–81
Rodriguez-Navarro JA, Rodriguez L, Casarejos MJ et al (2010) Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis 39:423–438
Neystat M, Lynch T, Przedborski S, Kholodilov N, Rzhetskaya M, Burke RE (1999) Alpha-synuclein expression in substantia nigra and cortex in Parkinson’s disease. Mov Disord 14:417–422
Wu J, Yu W, Chen Y et al (2010) Intrastriatal Transplantation of GDNF-engineered BMSCs and its neuroprotection in Lactacystin-induced Parkinsonian Rat Model. Neurochem Res 35:495–502
Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W (2008) Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis 32:16–25
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant {alpha}-Synuclein by Chaperone-mediated autophagy. Science 305:1292–1295
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29:528–535
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937
Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11:1107–1117
Galluzzi L, Morselli E, Vicencio JM et al (2008) Life, death and burial: multifaceted impact of autophagy. Biochem Soc Trans 36:786–790
Aguib Y, Heiseke A, Gilch S et al (2009) Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 5:361–369
Beranger F, Crozet C, Goldsborough A, Lehmann S (2008) Trehalose impairs aggregation of PrPSc molecules and protects prion-infected cells against oxidative damage. Biochem Biophys Res Commun 374:44–48
Olanow CW, Prusiner SB (2009) Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA 106:12571–12572
Minutoli L, Altavilla D, Bitto A et al (2008) Trehalose: a biophysics approach to modulate the inflammatory response during endotoxic shock. Eur J Pharmacol 589:272–280
Honda Y, Tanaka M, Honda S (2010) Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging Cell 9:558–569
Richards AB, Krakowka S, Dexter LB et al (2002) Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem Toxicol 40:871–898
Acknowledgments
This study was supported by the grants (30600663, 81071018) from the Natural Science Foundation of China, Project (10PJ1401700) of Pujiang Talents at Shanghai, project (09411960900) from Science and Technology Commission of Shanghai Municipality, and project (2010-116) of Shanghai Municipal Health Bureau.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dan-Mei Lan and Feng-Tao Liu have equal contribution to this work.
Rights and permissions
About this article
Cite this article
Lan, DM., Liu, FT., Zhao, J. et al. Effect of Trehalose on PC12 Cells Overexpressing Wild-Type or A53T Mutant α-synuclein. Neurochem Res 37, 2025–2032 (2012). https://doi.org/10.1007/s11064-012-0823-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0823-0