Abstract
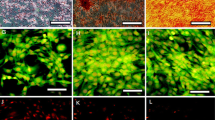
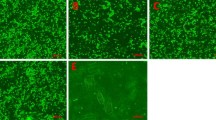
Muscle-derived stem cells reside in the skeletal muscle tissues and are known for their multipotency to differentiate toward the mesodermal lineage. Recent studies have demonstrated their capacity of neuroectodermal differentiation, including neurons and astrocytes. In this study, we investigated the possibility of dopaminergic neuronal conversion from adult rat skeletal muscle-derived stem cells. Using a neurosphere protocol, muscle-derived stem cells form neurosphere-like cell clusters after cultivation as a suspension, displaying an obvious expression of nestin and a remarkable down-regulation of myogenic associated factors desmin, MyoD, Myf5 and myogenin. Subsequently, these neurosphere-like cell clusters were further directed to dopaminergic differentiation through two major induction steps, patterning to midbrain progenitors with sonic hedgehog and fibroblast growth factor 8, followed by the differentiation to dopaminergic neurons with neurotrophic factors (glial cell line-derived neurotrophic factor) and chemicals (ascorbic acid, forskolin). After the differentiation, these cells expressed tyrosine hydroxylase, dopamine transporter, dopamine D1 receptor and synapse-associated protein synapsin I. Several genes, Nurr1, Lmx1b, and En1, which are critically related with the development of dopaminergic neurons, were also significantly up-regulated. The present results indicate that adult skeletal muscle-derived stem cells could provide a promising cell source for autologous transplantation for neurodegenerative diseases in the future, especially the Parkinson’s disease.




Similar content being viewed by others
References
Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122(2):289–301
Morgan JE, Partridge TA (2003) Muscle satellite cells. Int J Biochem Cell Biol 35(8):1151–1156
Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J (2005) Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell 16(7):3323–3333
Usas A, Maciulaitis J, Maciulaitis R, Jakuboniene N, Milasius A, Huard J (2011) Skeletal muscle-derived stem cells: implications for cell-mediated therapies. Medicina (Kaunas) 47(9):469–479
Deasy BM, Jankowski RJ, Huard J (2001) Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis 27(5):924–933
Huard J, Cao B, Qu-Petersen Z (2003) Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res C Embryo Today 69(3):230–237
Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R (2008) High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci USA 105(4):1226–1231
Sun JS, Wu SY, Lin FH (2005) The role of muscle-derived stem cells in bone tissue engineering. Biomaterials 26(18):3953–3960
Ye C, Li J, He Z, Nin X, Zhang Y, Shang X, Liu R, Duan Y (2010) Multilineage differentiation of muscle-derived stem cells from GFP transgenic mice. Biotechnol Lett 32(11):1745–1752
Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22(3):377–384
Arriero M, Brodsky SV, Gealekman O, Lucas PA, Goligorsky MS (2004) Adult skeletal muscle stem cells differentiate into endothelial lineage and ameliorate renal dysfunction after acute ischemia. Am J Physiol Renal Physiol 287(4):F621–F627
Bellayr IH, Gharaibeh B, Huard J, Li Y (2010) Skeletal muscle-derived stem cells differentiate into hepatocyte-like cells and aid in liver regeneration. Int J Clin Exp Pathol 3(7):681–690
Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J (2003) Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol 5(7):640–646
Farace F, Prestoz L, Badaoui S, Guillier M, Haond C, Opolon P, Thomas JL, Zalc B, Vainchenker W, Turhan AG (2004) Evaluation of hematopoietic potential generated by transplantation of muscle-derived stem cells in mice. Stem Cells Dev 13(1):83–92
Arsic N, Mamaeva D, Lamb NJ, Fernandez A (2008) Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res 314(6):1266–1280
Kim ES, Kim GH, Kang ML, Kang YM, Kang KN, Hwang KC, Min BH, Kim JH, Kim MS (2011) Potential induction of rat muscle-derived stem cells to neural-like cells by retinoic acid. J Tissue Eng Regen Med 5(5):410–414
Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E (2004) Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet 364(9448):1872–1883
Kondo T, Case J, Srour EF, Hashino E (2006) Skeletal muscle-derived progenitor cells exhibit neural competence. NeuroReport 17(1):1–4
Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Wada M, Hoshi A, Ishikawa T, Akatsuka A (2007) Clonal multipotency of skeletal muscle-derived stem cells between mesodermal and ectodermal lineage. Stem Cells 25(9):2283–2290
Anwar MR, Andreasen CM, Lippert SK, Zimmer J, Martinez-Serrano A, Meyer M (2008) Dopaminergic differentiation of human neural stem cells mediated by co-cultured rat striatal brain slices. J Neurochem 105(2):460–470
Cho MS, Hwang DY, Kim DW (2008) Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc 3(12):1888–1894
Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 99(4):2344–2349
Wang X, Li X, Wang K, Zhou H, Xue B, Li L (2004) Forskolin cooperating with growth factor on generation of dopaminergic neurons from human fetal mesencephalic neural progenitor cells. Neurosci Lett 362(2):117–121
Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D (2008) Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev 17(3):547–554
Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals JM, Memo M, Alberch J, Lopez-Barneo J, Vila M, Cuervo AM, Tolosa E, Consiglio A, Raya A (2012) Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med 4(5):380–395
Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X (2010) Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28(10):1893–1904
Fong CY, Gauthaman K, Bongso A (2010) Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem 111(4):769–781
Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K (2010) Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab 30(8):1487–1493
Romero-Ramos M, Vourc’h P, Young HE, Lucas PA, Wu Y, Chivatakarn O, Zaman R, Dunkelman N, el-Kalay MA, Chesselet MF (2002) Neuronal differentiation of stem cells isolated from adult muscle. J Neurosci Res 69(6):894–907
Rando TA, Blau HM (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125(6):1275–1287
Shen JL, Huang YZ, Xu SX, Zheng PH, Yin WJ, Cen J, Gong LZ (2012) Effectiveness of human mesenchymal stem cells derived from bone marrow cryopreserved for 23–25 years. Cryobiology (Epub ahead of print)
Tsai MS, Lee JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19(6):1450–1456
Jackson KA, Mi T, Goodell MA (1999) Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 96(25):14482–14486
Telford WG, Bradford J, Godfrey W, Robey RW, Bates SE (2007) Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells 25(4):1029–1036
Asakura A, Rudnicki MA (2002) Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30(11):1339–1345
Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7(9):1028–1034
Kachinsky AM, Dominov JA, Miller JB (1994) Myogenesis and the intermediate filament protein, nestin. Dev Biol 165(1):216–228
Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD (2000) Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 18(6):675–679
Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A (1998) FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93(5):755–766
Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog Neurobiol 77(1–2):128–138
Vourc’h P, Romero-Ramos M, Chivatakarn O, Young HE, Lucas PA, El-Kalay M, Chesselet MF (2004) Isolation and characterization of cells with neurogenic potential from adult skeletal muscle. Biochem Biophys Res Commun 317(3):893–901
Wu X, Wang S, Chen B, An X (2010) Muscle-derived stem cells: isolation, characterization, differentiation, and application in cell and gene therapy. Cell Tissue Res 340(3):549–567
Baek YS, Kang SH, Park JS, Kim S, Yoo BS, Lee JY, Ghil SH (2009) Long-term cultured skeletal muscle-derived neural precursor cells and their neurogenic potentials. NeuroReport 20(12):1109–1114
Lawson-Smith MJ, McGeachie JK (1998) The identification of myogenic cells in skeletal muscle, with emphasis on the use of tritiated thymidine autoradiography and desmin antibodies. J Anat 192(Pt 2):161–171
Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O (1998) Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim Biophys Acta 1402(1):39–51
Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A (1994) Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199(4):326–337
Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS (1999) In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci 112(Pt 17):2895–2901
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60(4):585–595
Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z (2004) Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci 117(Pt 22):5393–5404
Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA (1998) A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods 85(2):141–152
Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418(6893):50–56
Laywell ED, Kukekov VG, Steindler DA (1999) Multipotent neurospheres can be derived from forebrain subependymal zone and spinal cord of adult mice after protracted postmortem intervals. Exp Neurol 156(2):430–433
Neumeister B, Grabosch A, Basak O, Kemler R, Taylor V (2009) Neural progenitors of the postnatal and adult mouse forebrain retain the ability to self-replicate, form neurospheres, and undergo multipotent differentiation in vivo. Stem Cells 27(3):714–723
Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN (2001) Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol 19(5):475–479
Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A, Galli R (1999) Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol 156(1):71–83
Chen X, Mao Z, Liu S, Liu H, Wang X, Wu H, Wu Y, Zhao T, Fan W, Li Y, Yew DT, Kindler PM, Li L, He Q, Qian L, Fan M (2005) Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro. Mol Biol Cell 16(7):3140–3151
Riazi AM, Lee H, Hsu C, Van Arsdell G (2005) CSX/Nkx2.5 modulates differentiation of skeletal myoblasts and promotes differentiation into neuronal cells in vitro. J Biol Chem 280(11):10716–10720
Smidt MP, Burbach JP (2007) How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci 8(1):21–32
Prakash N, Wurst W (2006) Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci 63(2):187–206
Simon HH, Bhatt L, Gherbassi D, Sgado P, Alberi L (2003) Midbrain dopaminergic neurons: determination of their developmental fate by transcription factors. Ann NY Acad Sci 991:36–47
Schultz SS, Lucas PA (2006) Human stem cells isolated from adult skeletal muscle differentiate into neural phenotypes. J Neurosci Methods 152(1–2):144–155
Acknowledgments
This work was supported by grants from the Chinese National Basic Research 973 Program (2011CB504100, 2010CB944801) and National Science Foundation of Beijing (5102010). We thank Dr. Yang Li for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Wang, X., Wang, Y. et al. Dopaminergic Neuronal Conversion from Adult Rat Skeletal Muscle-Derived Stem Cells In Vitro. Neurochem Res 37, 1982–1992 (2012). https://doi.org/10.1007/s11064-012-0819-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0819-9