Abstract
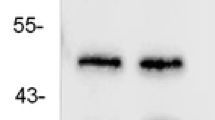
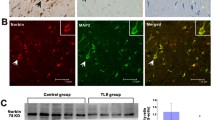
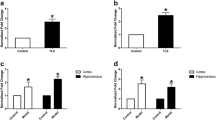
Methyl CpG binding protein-2 (MeCP2) is a multifunctional nuclear protein, and regulates dendritic morphology, synaptic transmission, spontaneous neurotransmission, and short-term synaptic plasticity in the central nervous system. This study was designed to investigate the expression of MeCP2 mRNA and protein in intractable temporal lobe epilepsy (TLE) patients and an experimental animal model. MeCP2 expression was detected in 35 temporal neocortex tissue samples from patients with intractable TLE and 14 histologically normal temporal lobe tissue samples from trauma patients without epilepsy by reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry and double-label immunofluorescence. In addition, the timing of MeCP2 expression was evaluated in the hippocampus and adjacent cortex of lithium chloride/pilocarpine-induced TLE rats and uninduced controls. MeCP2 was found to be expressed mainly in the nuclei of neurons, and not expressed in astrocytes. MeCP2 expression was significantly higher in the TLE patients and rats than in the control groups. Following seizures in the rat model, MeCP2 expression gradually increased in the hippocampus and adjacent cortex during the acute period (days 1 and 2) and the latent period (days 7 and 14), but decreased during the chronic period (days 30 and 60). Up-regulated expression of MeCP2 in intractable TLE patients and experimental animals suggested that MeCP2 may be involved in the pathogenesis of TLE.






Similar content being viewed by others
Abbreviations
- AEDs:
-
Antiepileptic drugs
- CNS:
-
Central nervous system
- CREB:
-
cAMP response element-binding protein
- CT:
-
Computed tomography
- EEG:
-
Electroencephalogram
- FITC:
-
Fluorescein isothiocyanate
- GFAP:
-
Glial fibrillary acidic protein
- GluR2:
-
Glutamate receptor 2
- HDACs:
-
Histone deacetylases
- IE:
-
Intractable epilepsy
- i.p.:
-
Intraperitoneal
- LiCl:
-
Lithium chloride
- LTD:
-
Long-term depression
- LTP:
-
Long-term potentiation
- MBD:
-
Methyl-CpG-binding domain
- MeCP2:
-
Methyl CpG binding protein-2
- MFS:
-
Mossy fiber sprouting
- MRI:
-
Magnetic resonance imaging
- Neun:
-
Neuron nuclei
- NSE:
-
Neuron-specific enolase
- PI:
-
Propidium iodide
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- RTT:
-
Rett syndrome
- SABC:
-
Streptavidin biotin complex
- TLE:
-
Temporal lobe epilepsy
- TRD:
-
Transcriptional repression domain
- TRITC:
-
Tetramethylrhodamine isothiocyanate
References
Regesta G, Tanganelli P (1999) Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res 34(2–3):109–122. doi:10.1016/S0920-1211(98)00106-5
Scharfman HE (2007) The neurobiology of epilepsy. Curr Neurol Neurosci Rep 7(4):348–354. doi:10.1007/s11910-007-0053-z
Rampp S, Stefan H (2006) Fast activity as a surrogate marker of epileptic network function? Clin Neurophysiol 117(10):2111–2117. doi:10.1016/j.clinph.2006.02.023
Dudek FE, Patrylo PR, Wuarin JP (1999) Mechanisms of neuronal synchronization during epileptiform activity. Adv Neurol 79:699–708
Cloix JF, Hevor T (2009) Epilepsy, regulation of brain energy metabolism and neurotransmission. Curr Med Chem 16(7):841–853. doi:10.2174/092986709787549316
McCormick DA, Contreras D (2001) On the cellular and network bases of epileptic seizures. Annu Rev Physiol 63:815–846. doi:10.1146/annurev.physiol.63.1.81563/1/815
Scharfman HE, McCloskey DP (2009) Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res 85(2–3):150–161. doi:10.1016/j.eplepsyres.2009.03.006
Leite JP, Neder L, Arisi GM, Carlotti CG Jr, Assirati JA, Moreira JE (2005) Plasticity, synaptic strength, and epilepsy: what can we learn from ultrastructural data? Epilepsia 46(Suppl 5):134–141. doi:10.1111/j.1528-1167.2005.01021.x
Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393(6683):386–389. doi:10.1038/30764
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19(2):187–191. doi:10.1038/561
Hite KC, Adams VH, Hansen JC (2009) Recent advances in MeCP2 structure and function. Biochem Cell Biol 87(1):219–227. doi:10.1139/o08-115
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23(2):185–188. doi:10.1038/13810
Glaze DG, Percy AK, Skinner S, Motil KJ, Neul JL, Barrish JO, Lane JB, Geerts SP, Annese F, Graham J, McNair L, Lee HS (2010) Epilepsy and the natural history of Rett syndrome. Neurology 74(11):909–912. doi:10.1212/WNL.0b013e3181d6b852
Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY (2002) Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet 11(2):115–124. doi:10.1093/hmg/11.2.115
Balmer D, Goldstine J, Rao YM, LaSalle JM (2003) Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J Mol Med 81(1):61–68. doi:10.1007/s00109-002-0396-5
Francke U (2006) Mechanisms of disease: neurogenetics of MeCP2 deficiency. Nat Clin Pract Neurol 2(4):212–221. doi:10.1038/ncpneuro0148
Na ES, Monteggia LM (2010) The role of MeCP2 in CNS development and function. Horm Behav. doi:10.1016/j.yhbeh.2010.05.014
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294
Fang M, Liu GW, Pan YM, Shen L, Li CS, Xi ZQ, Xiao F, Wang L, Chen D, Wang XF (2010) Abnormal expression and spatiotemporal change of Slit2 in neurons and astrocytes in temporal lobe epileptic foci: a study of epileptic patients and experimental animals. Brain Res 1324:14–23
Sperk G, Drexel M, Pirker S (2009) Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia 50(Suppl 12):29–31. doi:10.1111/j.1528-1167.2009.02365.x
Leite JP, Neder L, Arisi GM, Carlotti CG Jr, Assirati JA, Moreira JE (2005) Plasticity, synaptic strength, and epilepsy: what can we learn from ultrastructural data? Epilepsia 46(Suppl 5):134–141. doi:10.1111/j.1528-1167.2005.01021.x
Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM (2006) Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis 21(1):217–227. doi:10.1016/j.nbd.2005.07.005
Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY (2006) Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 26(1):319–327. doi:10.1523/JNEUROSCI.2623-05.2006
Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY (2004) Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet 13(21):2679–2689. doi:10.1093/hmg/ddh282ddh282
Chao HT, Zoghbi HY, Rosenmund C (2007) MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56(1):58–65. doi:10.1016/j.neuron.2007.08.018
Nelson ED, Kavalali ET, Monteggia LM (2006) MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol 16(7):710–716. doi:10.1016/j.cub.2006.02.062
Majores M, Schoch S, Lie A, Becker AJ (2007) Molecular neuropathology of temporal lobe epilepsy: complementary approaches in animal models and human disease tissue. Epilepsia 48(Suppl 2):4–12
Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM (2007) 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet 16(6):691–703. doi:10.1093/hmg/ddm014
Samaco RC, Hogart A, LaSalle JM (2005) Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet 14(4):483–492. doi:10.1093/hmg/ddi045
Williams PA, Hellier JL, White AM, Staley KJ, Dudek FE (2007) Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis. Epilepsia 48(Suppl 5):157–163. doi:10.1111/j.15281167.2007.01304.x
Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X (2007) Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis 27(1):77–89. doi:10.1016/j.nbd.2007.04.005
Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME (2006) Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52(2):255–269. doi:10.1016/j.neuron.2006.09.037
Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L (2009) Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis 35(2):219–233. doi:10.1016/j.nbd.2009.05.001
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320(5880):1224–1229. doi:10.1126/science.1153252
Acknowledgments
This work was supported by a grant from the Key Project of Chongqing Municipal Health Bureau for Medical Research (No. 2010-1-36) and the National Natural Science Foundation of China (No. 81171225). The authors sincerely thank the Beijing Tiantan Hospital, Xuanwu Hospital of Capital University of Medical Sciences, and Xin-hua Hospital of Hubei for supplying the brain tissues used in this study, the patients and their families for their participation in this study, and the National Board of the Medical Affairs and the local ethics committee.
Conflict of interest
The authors declare that they have no conflict of interests regarding the publication of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shuxin Tao and Xiaolan Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tao, S., Yang, X., Chen, Y. et al. Up-Regulated Methyl CpG Binding Protein-2 in Intractable Temporal Lobe Epilepsy Patients and a Rat Model. Neurochem Res 37, 1886–1897 (2012). https://doi.org/10.1007/s11064-012-0804-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0804-3