Abstract
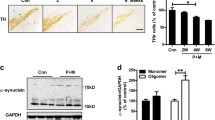
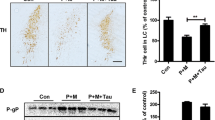
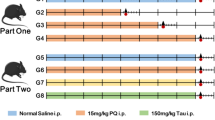
Maneb and paraquat are known to induce Parkinson’s disease (PD) phenotype, however, caffeine offers neuroprotection. Nitric oxide (NO) acts an important mediator in PD phenotype and tyrosine kinase (TK), nuclear factor kappa B (NF-kB), p38 mitogen activated protein kinase (p38 MAPK) are known to regulate its production. The present study aimed to elucidate the role of caffeine in the regulation of NO production and microglial activation and their subsequent contribution in dopaminergic neuroprotection. The animals were treated with caffeine and/or maneb and paraquat along with controls. In a few sets of experiments, the animals were also treated with aminoguanidine, an inhibitor of inducible NO synthase, pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-kB, genistein, an inhibitor of TK or SB202190, an inhibitor of p38 MAPK. Tyrosine hydroxylase (TH)-immunoreactivity and anti-integrin αM (OX-42) staining were performed to assess the number of dopaminergic neurons and activation of microglia, respectively. NO was measured in terms of nitrite, however, the expressions of p38 MAPK, interleukin (IL)-1β, NF-kB and TK were checked by western blot analyses. Maneb and paraquat induced the number of degenerating dopaminergic neurons, microglial cells, nitrite content, expressions of IL-1β, p38 MAPK, NF-kB and TK and caffeine co-treatment reduced the level of such alterations. Reductions were more pronounced in the animals co-treated with aminoguanidine, PDTC, genistein or SB202190. The results obtained thus demonstrate that caffeine down-regulates NO production, neuroinflammation and microglial activation, which possibly contribute to neuroprotection.





Similar content being viewed by others
References
Klockgether T (2004) Parkinson’s disease: clinical aspects. Cell Tissue Res 318:115–120
Singh MP, Patel S, Dikshit M, Gupta YK (2006) Contribution of genomics and proteomics in understanding the role of modifying factors in Parkinson’s disease. Indian J Biochem Biophys 43:69–81
Patt S, Gertz HJ, Gerhard L, Cervos-Navarro J (1991) Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: a Golgi study. Histol Histopathol 6:373–380
Tanner CM, Langston JW (1990) Do environmental toxins cause Parkinson’s disease? A critical review. Neurology 40:17–30
Thiruchelvam M, Richfield EK, Baggs RB et al (2000) The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci 20:9207–9214
Patel S, Singh V, Kumar A et al (2006) Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res 1081:9–18
Singh AK, Tiwari MN, Upadhyay G et al (2012) Long-term exposure to cypermethrin induces the nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging 33:404–415
Singh K, Singh S, Singhal NK et al (2010) Nicotine- and caffeine-mediated changes in gene expression patterns of MPTP-lesioned mouse striatum: implications in neuroprotection mechanism. Chem Biol Interact 185:81–93
Thrash B, Uthayathas S, Karuppagounder SS et al (2007) Paraquat and maneb induced neurotoxicity. Proc West Pharmacol Soc 50:31–42
Srivastava G, Singh K, Tiwari MN, Singh MP (2010) Proteomics in Parkinson’s disease: current trends, translational snags and future possibilities. Expert Rev Proteomics 7:127–139
Thiruchelvam M, Richfield EK, Goodman BM et al (2002) Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology 23:621–633
Patel S, Singh K, Singh S, Singh MP (2008) Gene expression profiles of mouse striatum in control and maneb + paraquat-induced Parkinson’s disease phenotype: validation of differentially expressed energy metabolizing transcripts. Mol Biotechnol 40:59–68
Kachroo A, Irizarry MC, Schwarzschild MA (2010) Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp Neurol 223:657–661
Gupta SP, Patel S, Yadav S et al (2010) Involvement of nitric oxide in maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: is there any link with lipid peroxidation? Neurochem Res 35:1206–1213
Cicchetti F, Lapointe N, Roberge-Tremblay A et al (2005) Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis 20:360–371
Fredholm BB, Ijzerman AP, Jacobson KA et al (2001) International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Ross GW, Abbott RD, Petrovitch H et al (2000) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283:2674–2679
Ascherio A, Zhang SM, Hernán MA et al (2001) Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol 50:56–63
Singh S, Singh K, Gupta SP et al (2009) Effect of caffeine on the expression of cytochrome P450 1A2, adenosine A2A receptor and dopamine transporter in control and 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine treated mouse striatum. Brain Res 1283:115–126
Singh S, Singh K, Patel S et al (2008) Nicotine and caffeine-mediated modulation in the expression of toxicant responsive genes and vesicular monoamine transporter-2 in 1-methyl 4-phenyl-1, 2, 3, 6-tetrahydropyridine’induced Parkinson’s disease phenotype in mouse. Brain Res 1207:193–206
Richardson PJ, Kase H, Jenner PG (1997) Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson-s disease. Trends Pharmacol Sci 18:338–344
Ochi M, Koga K, Kurokawa M et al (2000) Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience 100:53–62
Lindskog M, Svenningsson P, Pozzi L (2002) Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature 418:774–778
Chen X, Lan X, Roche I et al (2008) Caffeine protects against MPTP-induced blood–brain barrier dysfunction in mouse striatum. J Neurochem 107:1147–1157
Nakaso K, Ito S, Nakashima K (2008) Caffeine activates the PI3 K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett 432:146–150
Singhal NK, Srivastava G, Patel DK et al (2011) Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J Pineal Res 50:97–109
Singh AK, Tiwari MN, Dixit A et al (2011) Nigrostriatal proteomics of cypermethrin-induced dopaminergic neurodegeneration: microglial activation dependent and independent regulations. Toxicol Sci 122:526–538
Martin PY, Bianchi M, Roger F et al (2002) Arginine vasopressin modulates expression of neuronal NOS in rat renal medulla. Am J Physiol Renal Physiol 283:F559–F568
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
McCormack AL, Thiruchelvam M, Manning-Bog AB et al (2002) Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10:119–127
Patel S, Sinha A, Singh MP (2007) Identification of differentially expressed proteins in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse. Neurotoxicol Teratol 29:578–585
Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170
Kim WG, Mohney RP, Wilson B et al (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20:6309–6316
Wu XF, Block ML, Zhen W et al (2005) The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal 7:654–661
Zhou Y, Wang Y, Kovacs M et al (2005) Microglial activation induced by neurodegeneration: a proteomic analysis. Mol Cell Proteomics 4:1471–1479
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
Pott Godoy MC, Tarelli R, Ferrar CC et al (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 131:1880–1894
Qian L, Flood PM (2008) Microglial cells and Parkinson’s disease. Immunol Res 41:155–164
Hunot S, Brugg B, Ricard D et al (1997) Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci USA 94:7531–7536
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Cogswell JP, Godlevski MM, Wisely GB et al (1994) NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol 153:712–723
Wilms H, Rosenstiel P, Sievers J et al (2003) Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J 17:500–502
Acknowledgments
We sincerely acknowledge the CSIR, New Delhi, India for providing research fellowships to Sharawan Yadav, Satya Prakash Gupta and Garima Srivastava. This work was supported by the Department of Biotechnology, Government of India. The CSIR–IITR communication number of this article is 2971.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, S., Gupta, S.P., Srivastava, G. et al. Role of Secondary Mediators in Caffeine-Mediated Neuroprotection in Maneb- and Paraquat-Induced Parkinson’s Disease Phenotype in the Mouse. Neurochem Res 37, 875–884 (2012). https://doi.org/10.1007/s11064-011-0682-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0682-0