Abstract
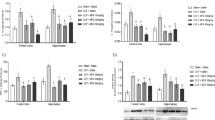
We verify the levels of cytokine/chemokine, myeloperoxidase activity, oxidative stress and disruption of BBB in hippocampus and cortex of the neonate Wistar rats after meningitis by S. agalactiae. In the hippocampus the levels were increased of CINC-1 at 6 h and 12 h, IL-1β at 6, 12 and 24 h, IL-6 at 6, 24 and 96 h, IL-10 at 24, 48 and 96 h and TNF-α at 24 h and 96 h. In the cortex the CINC-1 and IL-1β levels were found increased at 6 h. The MPO activity was significantly elevated at 24, 48 and 98 h in hippocampus and at 6, 12, 24, 48 and 96 h in the cortex. The breakdown of BBB started at 12 h.TBARS levels were elevated in the hippocampus at 6, 12, 24, 48, 72 and 96 h and cortex at 72 and 96 h. Protein carbonyls were elevated in the hippocampus and cortex at 6, 24, 48, 72 and 96 h. There was a decrease of SOD activity in hippocampus and in cortex. Catalase activity was elevated in hippocampus at 6 h and in the cortex at 12 and 96 h. Neonatal bacterial infections of the CNS are severe, the interference with the complex network of cytokines/chemokine, other inflammatory mediators and oxidants tend to aggravate the illness and can be involved in the breakdown of the BBB.







Similar content being viewed by others
Abbreviations
- BBB:
-
Blood-brain barrier
- CAT:
-
Calatase
- CFU:
-
Colony-forming unit
- CINC-1:
-
Cytokine-Induced neutrophil chemoattractant
- CSF:
-
Cerebrospinal fluid
- DNA:
-
Deoxyribonucleic acid
- DNPH:
-
Dinitrophenylhidrazine
- GBS:
-
Group B Streptococcus
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin 1 betha
- IL-10:
-
Interleukin 10
- ip:
-
Intra peritoneal
- MPO:
-
Myeloperoxidase activity
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive species
- TNF-α:
-
Tumor necrosis factor alpha
References
Pass MA, Gray BM, Dillon HC Jr (1982) Puerperal and perinatal infections with group B streptococci. Am J Obstet Gynecol 143:147–152
Phares CR, Lynfield R, Farley MM et al (2008) Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065
Tumbaga PF, Philip AG (2006) Perinatal Group B streptococcal infections and the new Guidelines: an update. Neoreviews 7:530–534
Lindahl G, Stålhammar-Carlemalm M, Areschoug T (2005) Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18:102–127
Sellner J, Täuber MG, Leib SL (2010) Pathogenesis and pathophysiology of bacterial CNS infections. Handb Clin Neurol 96:1–16
Verani JR, McGee L, Schrag SJ (2010) Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Prevention of perinatal group B streptococcal disease–revised guidelines from CDC. MMWR Recomm Rep 59:1–36
Lowry OH, Rosebrough NJ, Farr AL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Coimbra RS, Loquet G, Leib SL (2007) Limited efficacy of adjuvant therapy with dexamethasone in preventing hearing loss due to experimental pneumococcal meningitis in the infant rat. Pediatr Res 62:291–294
Maisey HC, Doran KS, Nizet V (2008) Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med 10:27
Bolduc GR, Baron MJ, Gravekamp C et al (2002) The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell Microbiol 4:751–758
Kim KS (2008) Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol 6:625–634
Yadav A, Malik GK, Trivedi R (2009) Correlation of CSF neuroinflammatory molecules with leptomeningeal cortical subcortical white matter fractional anisotropy in neonatal meningitis. Magn Reson Imaging 27:214–221
Moreillon P, Majcherczyk PA (2003) Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand J Infect Dis 35:632–641
van Furth AM, Roord JJ, van Furth R (1996) Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun 64:4883–4890
Miric D, Katanic R, Kisic B, Zoric L, Miric B, Mitic R, Dragojevic I (2010) Oxidative stress and myeloperoxidase activity during bacterial meningitis: effects of febrile episodes and the BBB permeability. Clin Biochem 43(3):246–252
Grandgirard D, Leib SL (2010) Meningitis en Neonatos: bench to bedside. Clin Perinatol 37:655–676
Edwards MS, Rench MA, Haffar AA et al (1985) Long-term sequelae of group B streptococcal meningitis in infants. J Pedriatr 106:717–722
Harvey D, Holt DE, Bedford H (1999) Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol 23:218–225
Johri AK, Paoletti LC, Glaser P et al (2006) Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol 4:932–942
Grandgirard D, Steiner O, Täuber MG et al (2007) An infant mouse model of brain damage in pneumococcal meningitis. Acta Neuropathol 114:609–617
Trampuz A, Steinhuber A, Wittwer M et al (2007) Rapid diagnosis of experimental meningitis by bacterial heat production in cerebrospinal fluid. BMC Infect Dis 7:116
Barichello T, Belarmino E, Comim CM et al (2010) Correlation between behavioral deficits and decreased brain-derived neurotrophic [corretion of neutrofic] factor in neonatal meningitis. J Neuroimmunol 233:73–76
Barichello T, Savi GD, Silva GZ et al (2010) Antibiotic therapy prevents, in part, the oxidative stress in the rat brain after meningitis induced by Streptococcus pneumoniae. Neurosci Lett 478:93–96
Kim KS (2010) Acute bacterial meningitis in infants and children. Lancet Infect Dis 10:32–42
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–341
Smith SL, Hall ED (1996) Mild pre- and posttraumatic hypothermia attenuates blood-brain barrier damage following controlled cortical impact injury in the rat. J Neurotrauma 13:1–9
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Leib SL, Tauber MG (1999) Pathogenisis of bacterial meningitis. Infect Dis Clin North Am 13:527–548
Hirst RA, Kadioglu A, O’callaghan C et al (2004) The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol 138:195–201
Klein M, Koedel U, Pfister HW (2006) Oxidative stress in pneumococcal meningitis: a future target for adjunctive therapy? Prog Neurobiol 80:269–280
Rusconi F, Parizzi F, Garlaschi L et al (1991) Interleukin 6 activity in infants and children with bacterial meningitis. The Collaborative Study on Meningitis. Pediatr Infect Dis J 10:117–121
Rosenberg GA, Estrada EY, Dencoff JE et al (1995) Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res 703:151–155
Kim KS, Wass CA, Cross AS (1997) Blood-brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp Neurol 145(1):253–257
Barichello T, Pereira JS, Savi GD et al (2011) A kinetic study of the cytokine/chemokines levels and disruption of blood-brain barrier in infant rats after pneumococcal meningitis. J Neuroimmunol 233(1–2):12–17
Zhang R, Brennan ML, Shen Z et al (2002) Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 277(48):46116–46122
Meli DN, Christen S, Leib SL (2003) Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway. J Infect Dis 187(9):1411–1415
Schaper M, Gergely S, Lykkesfeldt J et al (2002) Cerebral vasculature is the major target of oxidative protein alterations in bacterial meningitis. J Neuropathol Exp Neurol 61:605–613
Koracevic D, Koracevic G, Djordjevic V et al (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361
Leib SL, Kim YS, Chow LL et al (1996) Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest 98(11):2632–2639
Leib SL, Kim YS, Black SM et al (1998) Inducible nitric oxide synthase and the effect of aminoguanidine in experimental neonatal meningitis. J Infect Dis 177(3):692–700
Pfister HW, Koedel U, Dirnagl U et al (1990) Superoxide dismutase inhibits brain oedema formation in experimental pneumococcal meningitis. Acta Neurochir 51:378–380
Pfister HW, Ködel U, Dirnagl U et al (1992) Effect of catalase on regional cerebral blood flow and brain edema during the early phase of experimental pneumococcal meningitis. J Infect Dis 166:1442–1445
Ritter C, Andrades ME, Reinke A et al (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Barichello T, Fortunato JF, Vitali AM et al (2006) Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 34(3):886–889
Tyler KL (2008) Bacterial meningitis: an urgent need for further progress to reduce mortality and morbidity. Neurology 70:2095–2096
Acknowledgments
This research was supported by grants from CNPq, FAPEMIG, FAPESC, UNESC and Instituto Nacional de Ciência e Tecnologia Translacional em Medicina (INCT-TM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barichello, T., Lemos, J.C., Generoso, J.S. et al. Oxidative Stress, Cytokine/Chemokine and Disruption of Blood–Brain Barrier in Neonate Rats After Meningitis by Streptococcus agalactiae . Neurochem Res 36, 1922–1930 (2011). https://doi.org/10.1007/s11064-011-0514-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0514-2