Abstract
It is well established that lead (Pb) exposure in humans leads to learning and memory impairment. However, the biological and molecular mechanisms are still not clearly understood. When over activated, serine/threonine protein phosphatases are known to function as a constraint on learning and memory. Activation of these phosphatases can also result in cytoskeletal changes that will adversely affect learning and memory. We investigated the effects of Pb exposure on these phosphatases in primary cultures of human neurons. Neurons were exposed to physiologically relevant concentrations of Pb (5, 10, 20 and 40 μg/dL) and total phosphatase and PP2A activities were determined in neuronal lysate using para-nitrophenyl phosphate (pNPP), and a PP2A-specific phosphopeptide as substrates. Expression of various serine/threonine phosphatases, tau and its phosphorylation state were determined by Western blot (WB) and immunocytochemistry (ICC). We found that the total phosphatase activity in the neuronal lysate was increased by 30–50% by all the concentrations of Pb tested. PP2A activity was increased by 5 μg/dL Pb only. PP1 expression was increased (ranging from 25–50%) by 10, 20 and 40 μg/dL of Pb. PP2B expression was increased substantially (up to 2.5-fold) by 10 μg/dL Pb, whereas, higher concentrations did not show any effect. On the other hand, Pb (at all concentrations used) decreased expression of PP2A and PP5. Pb exposure induced substantial hyperphosphorylation of tau at serine 199/202 by 5 and 10 μg/dL Pb, and Threonine 231 at higher doses. Expression of total tau was mostly unaffected by lead. Immunocytochemistry data confirmed the WB results of expression of PP1, PP2A, tau protein and the phosphorylation of tau. These results support our hypothesis that Pb exposure up regulates some of the serine/threonine phosphatases (PP1 and PP2B) that are known to impair memory formation, and suggest a novel mechanism of Pb neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a toxic heavy metal that can affect several organs including the nervous, hematopoietic, renal, reproductive, endocrine and skeletal systems [1, 2]. The central nervous system (CNS) is particularly sensitive to Pb toxicity and is affected even by low doses [3, 4]. Lead exposure is associated with growth retardation in children [5, 6]. Exposure to low levels of Pb (as low as 7 μg/dL) is associated with impairment of cognitive and behavioural development in children [7, 8] and in young adults [9]. There is strong evidence that low-dose Pb exposure is causally associated with deficits in cognition [10]. An estimated decrease of 4–7 points in the mean IQ score for each 10 μg/dL increase in blood Pb has been reported [10].
Pb preferentially distributes in specific areas of the brain with the highest levels observed in the hippocampus and cerebral cortex—areas that are associated with learning and memory [11]. The exact mechanism(s) by which lead get into the body, and particularly into the brain, is still unknown as no Pb specific transporter(s) has been identified. However, it has been postulated that Pb might use the divalent metal ion transporter 1 (DMT1), which is used by several essential elements [12]. DMT1 is localized in astrocytes and neurons in the normal brain [13–15]. The developing brain is a primary target for lead-induced neurotoxicity. However, the mechanism of lead-induced neurotoxicity and deficits in learning and memory is not well understood. Neurogenesis and synaptogenesis are the main components of learning and memory and these are both known to be affected by Pb [16–19].
Protein phosphorylation/dephosphorylation is one of the key events in the regulation of several cellular events including synaptogenesis. Reversible protein phosphorylation is the result of a delicate coordinated balance between protein kinases and protein phosphatases. The major serine/threonine protein phosphatases in the brain are PP1, PP2A and PP2B [20, 21]. These phosphatases, together with protein kinases, contribute to the control of synaptic plasticity and memory [22–27]. In the last decade, researchers have clearly demonstrated the importance of protein kinases in short and long-term modification of signal transmission between synapses. However, the roles of protein phosphatases have been appreciated recently and need to be further investigated.
Over-expression/over-activation of some of serine/threonine protein phosphatases has been associated with suppression of learning and memory. The role of PP1 in learning and memory has been widely studied [28, 29]. Inhibition of PP1 activity, either genetically or pharmacologically, enhanced learning capacity, suggesting that PP1 is a constraint on memory. This enhancement in learning was correlated with increased phosphorylation of several synaptic and nuclear targets of PP1 including GluR1, CaMKII, and CREB proteins. After learning, inhibition of PP1 prolonged memory, suggesting that PP1 also promotes forgetting. Thus PP1 appears to be a suppressor of learning and memory [30–33]. It has been suggested that PP1 determines the efficacy of learning and memory by limiting acquisition of information and favouring memory decline. When PP1 is inhibited, short intervals between training episodes are sufficient for optimal performance [31]. In addition to PP1, PP2A and PP2B are also known to adversely affect learning and memory [23, 32, 34, 35]. Mice over-expressing calcineurin (PP2B) have normal short-term memory but have profound and specific defect in long-term (hippocampal dependent) memory. The specific defects in long-term memory may be due to a defect in transition between short-term and long-term memory [23]. The effect of Pb exposure on the activity or expression of these phosphatases thus far has not been investigated in humans or in animal models.
An intact microtubule structure is essential for normal neuronal functioning. Disruption of microtubules has been shown to be associated with memory loss and neuronal death [36]. The microtubule network is stabilized by several microtubule-associated proteins (MAP); tau is one of them. Tau can be phosphorylated at several serine or threonine residues [37]. Phosphorylation of tau at a stoicheometry of 2–3 mol phosphates per mole of protein is required for promoting assembly and stability of the microtubule, whereas, its hyperphosphorylation (9–10 mol of phosphate per mole of tau) results in the disruption and disassembly of microtubule and subsequently in memory loss and neuronal death [38–41]. As the phosphorylation state of tau is regulated by the balance between protein kinases and protein phosphatises, over-expression and/or over-activation of phosphatases (PP1, PP2A or PP2B) will affect phosphorylation level of tau and thus the stability of microtubule.
In this study, we tested the hypotheses that exposure of human neurons to Pb results in over-expression or over-activation of serine/threonine protein phosphatases. The results presented here support this hypothesis and suggest a novel mechanism of lead-induced impairment of learning and memory, as these phosphatases are known to directly impair learning and memory.
Materials and Methods
Reagents and Chemicals
All neuronal cell culture media and additives were from Invitrogen (Mulgrave, VIC, Australia) unless otherwise stated. All chemical used for Western blot, immunoprecipitation, PPase activity and immunoblot (unless mentioned) were obtained from Sigma-Aldrich Chemical Co. (Sydney, NSW, Australia). The list of primary antibodies and their sources is given in Table 1. Prof Alistair Sim (University of Newcastle, NSW, Australia) kindly provided the PP1 antibody (PP1c). Secondary anti-mouse and anti-rabbit IgG and anti-rabbit Alexa 594 (Red)-conjugated antibodies were purchased from Molecular probes (Eugene, OR, USA). All commercial antibodies were used at the concentrations recommended by the manufacturer.
Cell Cultures
Human foetal brains were obtained from 16 to 19 week old foetuses collected following therapeutic termination with informed consent (Ethic approval HREC03187). Primary cultures of human neurons were prepared as described previously [42]. Briefly, cells were plated in 12-well tissue culture plates coated with Matrigel (diluted 1/20 in Neurobasal medium) and maintained in Neurobasal medium supplemented with 2% (v/v) B-27 supplement, 2 mM Glutamax, 50 mM HEPES, 200 IU/mL penicillin G, and 200 μg/mL streptomycin sulphate and 5 mM glucose. Cultures were kept at 37°C at 5% CO2 in a humidified atmosphere. The medium was changed once to twice per week. We used 3 week-old highly pure neuronal cultures for the experiments.
Preparation of Neuronal Lysate for Western Blots
For Western Blots, cells were cultured in Matrigel coated 12-well plates at a density of 1 × 106 cells per well in supplemented Neurobasal medium (as described above). Neurons were cultured with 5, 10, 20 and 40 μg/dL of elemental Pb for 72 h. A 10 μg/mL Pb solution was prepared by dissolving 183.07 mg of Pb(II) acetate trihydrate (Pb(C2H3O2)2·3H2O; FW 379.33) in 10 mL of deionised and sterilized water, taking into account the MW of lead as 207.20. Control cultures were kept in the medium alone. Cells were lysed in 500 μL of RIFA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 5 mM EDTA, 0.5% sod. deoxycholate, 0.1% SDS, 50 nM NaF, 1 mM sodium vanadate, and protease inhibitor cocktail (Roche Diagnostic, Castle Hill, NSW, Australia). The cell lysate was centrifuged at 16,000g for 10 min. Protein concentration in the supernatant was determined by the Bradford method (Sigma) and stored at −20°C until used.
SDS–PAGE and Immunoblot Analyses
20 μg lysate protein was resolved on 10% SDS–PAGE (NuPAGE 10% from Invitrogen, Carlsbad CA USA) in sample buffer. Protein was transferred onto PVDF membranes; the membranes were blocked with 5% milk in TBS for 1 h and incubated with primary antibody overnight at 4°C. The primary antibodies used are described in Table 1. After incubation, the membranes were rinsed several times, washed with TBS-tween three times (10 min each), and then two times with TBS only. The membranes were then incubated with the secondary antibody for 4 h at RT, washed as before and developed with ECL (Biorad, Gladesville, NSW, Australia) according to the manufacturer instructions. Blots were scanned and quantified using the software ImageJ from NIH (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2007).
Phosphatase Activity Assay
For phosphatase activity, cells were cultured as described above and lysed in the phosphatase activity buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM PMSF, 1 mM Benzamidine, 10 mM beta-mercaptoethanol (βME), and protease inhibitor cocktail. Total phosphatase activity was determined using pNPP (10 mM) as a substrate in 50 mM Tris–HCl, pH 7.4, 0.1 mg/mL BSA, 3 mM CaCl2, 1 μM calmodulin and 3 mM MnCl2. The reaction mixture was incubated at 30°C for 30 min and the absorbance measured at 405 nm. PP2A activity was indirectly measured by using 10 nM okadaic acid (OA) in the phosphatase assay buffer and then calculating the OA-inhibited activity.
Direct Estimation of PP2A Activity
Cells were cultured with various concentrations of Pb for 24 h. Cells were harvested and the lysate prepared in 20 mM imidazole–HCl, pH 7.0, 2 mM EDTA, 2 mM EGTA, and the protease inhibitor cocktail. PP2A activity was measured with the PP2A immunoprecipitation phosphatase assay kit (Cat. # 17-313; Millipore, Temecula, CA, USA) according the manufacturer protocol. Briefly, 200 μg neuronal lysate protein was immunoprecipitated with anti-PP2A (clone 1D6) and the activity of the immunoprecipitated PP2A was estimated by colorimetrically measuring the released phosphate from threonine phosphopeptide (K-R-pT-I-R-R) with Malachite Green phosphate detection solution.
Immunocytochemistry
The method was previously described [38]. Human foetal neurons were grown at the density 5 × 106 in Permanox chamber-slides (NUNC) for 2 weeks. Then, cells were fixed with acetone/methanol (vol/vol) for 20 min at −20°C. Cells were then rinsed three times with PBS and a gentle membranous permeabilization was performed by incubation with 0.025% Triton x100 in PBS for 10 min at room temperature. After washing, cells were incubated with 5% normal goat serum (NGS) in PBS for 45 min at room temperature, rinsed twice with PBS and incubated for 1 h at 37°C with selected primary antibodies diluted in 5% NGS. Antibodies used are Tau (for total tau), AT8 and AT-180, anti-PP1, anti-PP2A (clone 1D6). Cells were then washed with 5% NGS solution and incubated for 1 h at 37°C with the appropriate labelled secondary antibodies (Goat anti-mouse IgG or goat anti-rabbit coupled with Alexa 594). Nuclear staining was performed using DAPI at 1 μg/mL for 5 min at room temperature. After several washings with PBS at 37°C, the cover slips were quickly mounted on glass slides with Fluoromount-G, and were examined with an Olympus BX60 fluorescence microscope associated with a digital SensiCam. The following three controls were performed for each labelling experiment: (1) isotypic antibody controls for mAbs and serum control for pAbs, (2) incubation with only the secondary labelled antibodies, (3) estimation of auto-fluorescence of unlabelled cells. Fluorescence intensity was quantified using ImageJ 1.38 for Mac OSX (NIH, USA).
Statistical Analyses
Each experiment was repeated at least three times, unless otherwise stated. The number of replicates for each experiment is shown in the figure legends. Student t test of two independent samples with unequal variances was used to measure the significance level of the difference between control and various levels of Pb treatments. Significance level was set at P < 0.05.
Results
Lead Exposure Increases Phosphatase Activity in Human Neurons
To investigate the effect of Pb exposure on phosphatase activity in cultured primary human neurons, we cultured neurons with increasing concentrations of Pb for 72 h and measured phosphatase activity using pNPP as substrate. The results are shown in Fig. 1. All concentrations of Pb tested induced a significant increase (P < 0.05) in total phosphatase activity. The increase in phosphatise activity ranged from 30–46% compared to control levels but was not dose dependent (Fig. 1a). The PP2A activity, which was indirectly calculated as okadaic acid (10 nM)—inhibited activity, was substantially and significantly (P ≤ 0.01) increased (50%) with 5 μg/dL lead. Higher concentrations of Pb (10, 20 and 40 μg/dL) slightly decreased PP2A activity, but the decrease was not statistically significant (Fig. 1b). Direct measurement of PP2A activity with a PP2A activity assay kit, which involves the release of phosphate from threonine phosphopeptide (K-R-pT-I-R-R) by the immunoprecipitated PP2A, revealed similar results. In this case 5 μg/dL Pb significantly (P < 0.05) increased PP2A activity, whereas, higher concentrations of Pb did not significantly affect PP2A activity.
Lead affects protein phosphatase activities in cultured human neurons. a Neurons were cultured with various levels of Pb for 72 h. Cells were lysed in the phosphatase activity buffer. Total phosphatase activity was determined using pNPP (10 mM) as a substrate, as described in Material and Methods. Phosphatase activity of cultures treated with different concentrations of Pb is expressed as percent of the control cultures. Data presented are the mean ± SD of three experiments. b PP2A activity was indirectly measured in the presence of 10 nM OA in the phosphatase assay buffer. PP2A activity was calculated as the difference between total activity and activity in presence of OA (OA-inhibited activity). PP2A activity is the average of three experiments and expressed as percent of the control cultures. c PP2A was immunoprecipitated with anti-PP2A (clone 1D6) and the activity was determined as described in Materials and Methods. Data presented are the average of two separate experiments and the PP2A activity is expressed as the picomoles of phosphate released by PP2A from the phosphopeptide included in the kit. * significantly different from control (P < 0.05), ** significantly different from control (P < 0.01)
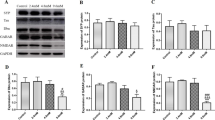
Lead exposure Dysregulates the Expression of Serine/Threonine Protein Phosphatases
We investigated the levels of protein expression of several serine/threonine protein phosphatases in the lysate of Pb-treated neurons. The results of these experiments are shown in Fig. 2. Pb at 10, 20 40 μg/dL significantly increased PP1 expression (P < 0.05); the highest increase (51%) was observed in neurons treated with 10 μg/dL Pb (Fig. 2a). Similarly, a 1.8 and 2.5-fold increase in PP2B expression was seen, respectively, in neurons treated with 5 and 10 μg/dL Pb, however, only the effect of 10 μg/dL Pb was statistically significant (P < 0.02). Higher concentrations did not show any effect on PP2B expression (Fig. 2b). Expression of PP2A was significantly (P < 0.01) decreased by all concentrations of Pb tested (Fig. 2c). PP5 expression was also decreased by Pb in a dose-dependent manner. Pb at 5 μg/dL decreased PP5 expression but the decrease was statistically not significant (P = 0.09). Higher concentrations of Pb significantly decreased PP5 expression (P < 0.01). PP5 was more sensitive to Pb than PP2A. Pb at 40 μg/dL decreased PP5 expression to less than 20% of control. The increase in PP1 expression and the decrease in PP2A expression with Pb treatments were confirmed with immunocytochemistry (Fig. 4).
Lead increases the expression of PP1and PP2B but decreases the expression PP2A and PP5. Primary foetal human neurons were cultured in Matrigel coated 12-well plates at a density of 1 × 106 cells per well with various concentrations of Pb for 72 h. Control cultures were kept in the medium alone. Cells were lysed in 500 μL of RIFA buffer, and 20 μg lysate protein was resolved on 10% SDS–PAGE and immunoblotted with antibodies to a PP1, b PP2A, c PP2B and d PP5. Blots were scanned and quantified using the software ImageJ from NIH. Anti-actin antibody was used as loading control to which data was normalized. Data are expressed as percent of control cultures and represent as mean ± SD of three experiment (for PP1 and PP2B) and six experiments (PP2A and PP5). Representative blots are shown with the graphs. * significantly different from control (P < 0.05), ** significantly different from control (P < 0.01)
Lead Exposure Affects the Phosphorylation State of Tau Protein
Since serine/threonine phosphatases modulate the rate of phosphorylation of the phosphoprotein tau, and Pb affects the activity and expression of these phosphatases, we investigated if Pb exposure would also affect the expression and phosphorylation of tau protein. All concentrations of Pb tested resulted in a modest increase in the expression of tau in a non-dose-dependent manner but the increase did not reach statistical significance (Fig. 3a). With regards to tau phosphorylation, Pb at 5 and 10 μg/dL resulted in significant increase in tau phosphorylation at the AT8 site. Higher concentration of Pb had no appreciable effect at tau phosphorylation at this site (Fig. 3b). At the AT-180 site, an increase in phosphorylation was observed with all levels of Pb tested but only the highest concentration (40 μg/dL) resulted in statistically significant increase in the phosphorylation of tau (Fig. 3c). We also tested the phosphorylation of tau at these two sites (AT8 and AT-180) by immunocytochemistry using 5 and 20 μg/dL Pb. Pb at 5 μg/dL substantially increased tau phosphorylation at AT8 and AT-180 sites, whereas, phosphorylation at these sites was not substantially affected by 20 μg/dL Pb (Fig. 4c, d). No significant effect of Pb on the expression of total tau was observed by immunocytochemistry, similar to the Western blot results (Fig. 4a).
Lead induces tau hyperphosphorylation in human neurons. Neurons were treated with various concentration of Pb for 72 h as described before. Cells were lysed in RIFA buffer and Western blot was performed using antibodies towards a total tau (tau), b tau phosphorylated at serine 199/202 (AT8) and c threonine 231 (AT180). Anti-actin antibody was used as loading control to which the data for total tau was normalized. The signal for AT8 and AT180 was normalized to the signal for total tau and is presented as arbitrary units. Quantitation shown is the average of two experiments from two separate neuronal cultures. Antibody dilution is shown in Table 1. *significantly different from control (P < 0.05), ** significantly different from control (P < 0.01)
Dysregulation of protein phosphatases and tau phosphorylation by lead in human neurons. Primary foetal human neurons were cultured in Matrigel coated 12-well plates at a density of 1 × 106 cells per well with 5 μg/dL and 20 μg/dL Pb for 24 h. Control cultures were kept in the medium alone. a Cultures were immunostained for total tau, tau phosphorylation with two different antibodies (AT8 and AT180), and PP1 and PP2A expression as described in Materials and Methods. The blue labelling with DAPI stained all cell nuclei and the red staining Tau. b Quantitative presentation of the immunohistochemistry staining. For each antibody used, the left column corresponds to untreated neurons, the middle column to 5 μg/dL Pb treated cells and the right column to 20 μg/dL Pb treated cells. The histograms represent the semi-quantification of the immunostaining intensity. Intensity has been quantified using ImageJ 1.38 for Mac OSX (NIH, USA). Three individual microscopic fields were analysed for each treatment and the SEM for the data was determined to be <5%. For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article. (mail as on 15/7/2006)
Discussion
We found that Pb in pathophysiological relevant concentrations increased total phosphatase activity and PP2A activity in primary cultures of human neurons. Pb also resulted in increased expression of PP1 and PP2B but it decreased the expression of PP2A and PP5. Pb substantially increased phosphorylation of serine 199/202 of tau (AT-8 site) particularly at lower doses (5 and 10 μg/dL), whereas, phosphorylation of threonine 231 was more pronounced at higher dose (40 μg/dL). These results suggest two possible mechanisms for Pb-induced impairment of learning and memory. First, Pb treatment (exposure) induces over-expression (PP1 and PP2B) and over-activation (PP2A) of serine/threonine protein phosphatases. Over-activation of these phosphatases is associated with learning and memory impairment [28–35]. Second, Pb exposure results in hyperphosphorylation of tau which may subsequently result in cytoskeletal disruption in neurons. Such structural changes are also reported to be responsible for memory loss in many tauopathies [38–41].
Our study is unique in three aspects. Firstly, we measured the activity and expression of PP1, PP2A and PP2B in primary cultures of human neurons. To our knowledge, this is the first study investigating the mechanism of Pb-induced changes at the molecular level in human neurons. Previous studies have been performed using animal models; however, these results may not be fully relevant for humans. Secondly, the effects of Pb exposure on brain phosphatases have not been studied before in humans or in animal models. Finally, we used pathophysiological levels of Pb that are in accordance with the blood levels of humans that have been exposed to environmental Pb contamination.
We found a 30–46% increase in total phosphatase activity in neurons treated with Pb for 72 h. Since PP2A accounts for the majority (~70%) of the brain phosphatase activity, we used two approaches the measure the activity of this phosphatase. (1) Okadaic acid at 10 nM concentration specifically inhibits PP2A activity without affecting PP1 or PP2B activity [43]. We used this approach to indirectly estimate PP2A activity. Using this method, we found that PP2A activity was particularly sensitive to Pb effect and we observed a significant increase (50%) but only with a small dose of Pb (5 μg/dL). Higher doses were not effective and resulted only in a limited (non-significant) decrease in PP2A activity. (2) A commercially available kit (Millipore, Temecula, CA, USA), which specifically measure PP2A activity was used. This technique involves the immunoprecipitation of PP2A with the anti-PP2A antibody (clone 1D6) and measuring the released phosphate by PP2A colorimetrically from a phosphopeptide. The results obtained from this approach were in perfect agreement with the okadaic acid-inhibited activity (Fig. 1b, c). From these results it can be confidently inferred that PP2A is more sensitive to lower doses of Pb and that such increase in PP2A activity by low doses of Pb may responsible for reported CNS toxicity [3, 4] and impairment of learning and memory [10] by low doses of Pb.
Several serien/threonine protein phosphatases are expressed in the brain. Of these, the major phosphatases in the brain are PP1, PP2A and PP2B [20, 21], with PP2A being the major one. PP2A (together with PP1) accounts for over 90% of the total mammalian brain protein phosphatase activity [44, 45]. PP2A alone also accounts for 71% of the brain tau phosphatase activity [46]. Our results suggest that different phosphatases are affected differently by Pb. We observed an increase in PP1 and PP2B expression but a decrease in the expression of PP2A and PP5. The increase in PP1 and PP2B expression may be a mechanism to compensate for the decrease in PP2A expression. Our results also suggest that the increase in total phosphatase activity, particularly at Pb doses higher than 5 μg/dL may be mainly due to an increase in PP1 and PP2B activity. However, this could not be verified as we did not individually determine the activities of PP1 and PP2B.
Treatment with low-level of Pb (5 μg/dL) resulted in increased activity of PP2A but a decrease of its expression. The reason for this apparent discrepancy between activity and expression of PP2A by Pb is not clear but could likely be explained by the complex structure and regulatory system of PP2A. PP2A is a trimeric protein consisting of a catalytic subunit C, a scaffolding subunit A and a regulatory subunit B. There are two isoforms of C, two isoforms of A and at least 18 isoforms of the regulatory subunit B. The expression levels of the A and C subunits are uniform throughout the brain but the expression levels of various regulatory subunits are highly variable depending upon cell types and tissues [47, 48]. Different combinatorial associations of the different A, B and the C subunits can generate 75 different trimeric PP2A holoenzymes. The activity of the holoenzyme towards different substrates is greatly modulated by the regulatory subunit B. These may modulate (1) the tissue localization and targeting the enzyme to particular cellular compartments, (2) the substrate specificity, and (3) catalytic activity toward the same substrate in response to different agents [47]. The C subunit as such is more active towards phosphoprotein substrates than is the core enzyme (Subunits A and C) in vitro, whereas it is the opposite for activity towards phosphopeptides [49, 50]. Similarly, the B subunits also modulate the catalytic activity of the core enzyme, suppressing activity towards some substrates, but greatly enhancing the dephosphorylation of others (reviewed by Janssens and Goris [47]). Whether Pb affects the association of the catalytic subunit of PP2A with other subunits or results in the selective expression of different regulatory subunits needs to be further investigated.
In addition to the direct impairment of learning and memory due to increase in the activity of phosphatases, Pb may also indirectly impair learning and memory by causing cytoskeletal changes in neurons by modulating the phosphorylation state of the tau protein. We observed a substantial increase in tau phosphorylation at two sites (serine 199/202 and threonine 231) in human neurons treated with various concentrations of Pb. An increase in the expression of phosphorylated tau in the hippocampus of mouse pups exposed to Pb in their early life has recently been reported [51]. Tau is a phosphoprotein that can be phosphorylated at multiple serine and threonine residues. Hyperphosphorylated tau is assembled into paired helical filaments (PHF) of neurofibrillary tangles [52, 53]. At least 30 serine/threonine phosphorylation sites have been identified in abnormally hyperphosphorylated tau [37]. Phosphorylation of tau at a stoicheometry of 2–3 mol phosphates per mole of protein is required for promoting assembly and stability of microtubule, whereas, its hyperphosphorylation (9–10 mol of phosphate per mole of tau) results in the disruption and disassembly of microtubule and subsequently in memory loss and neuronal death [36, 37, 41]. The disruption of microtubules in neurons by the hyperphosphorylated tau is one of the fundamental aspects of dementia in Alzheimer’s disease and other tauopathies [52–57]. Whether tau phosphorylation is involved in Pb-induced deficits in learning in young children is still unknown, and needs to be further investigated. However, several studies have shown that phosphorylated tau, irrespective of NFT accumulation, is responsible for memory deficits in many models of tauopathies (other than Alzheimer’s disease) such as Drosophila [58], mouse [59] and rat [60]. Based on these reports it would not be surprising if such mechanism also affect young children exposed to lead. The increase in tau phosphorylation at low dose of lead (5 μg/dL), particularly at serine 199/202 may be a mechanism for the reported impairment of learning and memory in children. This finding is in accordance with the published literature showing that Pb even at low doses (as low as <7 μg/dL) causes subtle alteration in synaptogenesis and impairs learning and memory, whereas, at high doses, it can cause encephalopathy [16, 61, 62].
In summary, we report here that Pb exposure, in pathophysiological concentrations, increases the activity and expression of different serine/threonine protein phosphatases. Pb exposure also induces hyperphosphorylation of tau at several sites. These data suggest that Pb may impair learning and memory by two mechanisms: (1) by up-regulating the activity (PP2A and possibly PP1 and PP2B) and expression (PP1 and PP2B) of protein phosphatases, which are known to impair learning and memory formation and (2) by inducing hyperphosphorylation of tau protein and the subsequent cytoskeletal changes in neurons.
References
Goyer RA (1995) Nutrition and metal toxicity. Am J Clin Nutr 61:646S–650S
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Rev 49:529–554
Bellinger DC (2008) Very low lead exposure and children’s neurodevelopment. Curr Opin Pediatr 20(2):172–177
Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Rev 27(2):168–176
Shukla R, Dietrick KN, Bornchein RL, Berger O, Hammond PB (1991) Lead exposure and growth in the early preschool child: a follow-up report from the Cincinnati Lead Study. Pediatrics 88:886–892
Schwartz J, Angle C, Pitcher H (1986) Relationship between childhood blood lead levels and stature. Pediatrics 77(3):281–288
Canfield RL, Kreher DA, Cornwell C, Nenderson CR Jr (2003) Low-level lead exposure, executive functioning, and learning in early childhood. Child Neuropsychol 9(1):35–53
Counter SA, Buchanan LH, Ortega F (2005) Neurocognitive impairment in lead-exposed children of Andean lead-glazing workers. J Occup Environ Med 47(3):306–312
Counter SA, Buchanan LH, Ortega F (2009) Neurocognitive screening of lead-exposed Andean adolescents and young adults. J Toxicol Environ Health A 72(10):625–632
Needleman HL, Gatsonis G (1990) Low-level lead exposure and the IQ of children: a meta analysis of modern studies. J Am Med Assoc 263:673–678
Lefauconnier JM, Bernard G, Mellerio F, Sebille A, Cesarini E (1983) Lead distribution in the nervous system of 8-month-old rats intoxicated since birth by lead. Experientia 39(9):1030–1031
Ong WY, He X, Chua LH, Ong CN (2006) Increased uptake of divalent metals lead and cadmium into the brain after kainite-induced neuronal injury. Exp Brain Res 173(3):468–474
Burdo JR, Martin J, Menzies SL, Dolan KG, Romano MA, Fletcher RJ, Garrick MD, Garrick LM, Connor JR (1999) Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience 93:1189–1196
Huang E, Ong WY, Connor JR (2004) Distribution of divalent metal transporter-1 in the monkey basal ganglia. Neuroscience 128:487–496
Wang XS, Ong WY, Connor JR (2001) A light and electron microscopic study of the iron transporter protein DMT-1 in the monkey cerebral neocortex and hippocampus. J Neurocytol 30:353–360
Gilbert ME, Kelly ME, Samsam TE, Coodman JH (2005) Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci 86(2):365–374
Jaako-Movits K, Zharkovsky T, Romantchik O, Jurgenson M, Merisalu E, Heidmets LT, Zharkovsky A (2005) Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J Dev Neurosci 23(7):627–635
McCauley PT, Bull RJ, Tonti AP, Lutkenhoff SD, Meister MV, Doerger JU, Stober JA (1982) The effect of prenatal and postnatal lead exposure on neonatal synaptogenesis in rat cerebral cortex. J Toxicol Environ Health 10(4–5):639–651
Verina T, Rohde CA, Guilarte TR (2007) Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145(3):1037–1047
Cohen P (1989) The structure and regulation of protein phosphatases. Annu Rev Biochem 58:453–508
Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22(8):1942–1950
Mulkey RM, Endo S, Shenolikar S, Malenka RC (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369:486–488
Mansuy IM, Mayfor M, Jacob B, Kandel ER, Bach ME (1998) Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 92(1):39–49
Mansuy IM, Shenolikar S (2006) Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci 29(12):679–686
Yamashita T, Inui S, Maeda K, Hua DR, Takagi K, Fukunaga K, Sskaguchi N (2006) Regulation of CamKII by alpha4/PP2Ac contributes to learning and memory. Brain Res 1082(1):1–10
Monti B, Berteotti C, Contestabile A (2005) Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus 15(8):1041–1049
Runyan JD, Moore AN and Dash PK (2005) A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory
Munton RP, Vizi S, Mansuy IM (2004) The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett 567:121–128
Jouvenceau A, Hedou G, Potier B, Kollen M, Dutar P, Mansuy IM (2006) Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci 24(2):564–572
Genoux C, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM (2002) Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418(6901):970–975
Bennett PC, Zhao W, Ng KT (2001) Concentration-dependent effects of protein phosphatase (PP) inhibitors implicate PP1 and PP2A in different stages of memory formation. Neurobiol Learn Mem 75(1):91–110
Bennett PC, Moutsoulas P, Lawen A, Perini E, Ng KT (2003) Novel effects on memory observed following unilateral intracranial administration of okadaic acid, cyclosporine A, FK506 and [MeVal4]CyA. Brain Res 988(1–2):56–68
Waddell S (2003) Protein phosphatase 1 and memory: practice makes PP1 imperfect? Trends Neurosci 26(3):117–119
Havekes R, Nijholt IM, Luiten PG, Van der Zee EA (2006) Differential involvement of hippocampal calcineurin during learning and reversal learning in a Y-maze task. Learn Mem 13(6):753–759
Bennett PC, Zhao W, Lawen A, Ng KT (1996) Cyclosporin A, an inhibitor of calcineurin, impairs memory formation in day-old chicks. Brain Res 730:107–117
Iqbal K, Alonso del AC, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Li F, Rahman A, Tanimukai H, Grundke-Iqbal I (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochem Biophys Acta 173:198–210
Gong CX, Liu F, Grundke-Iqbal I, Iqbal K (2005) Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm 112(6):813–838
Kopke E, Tung YC, Shaikh S, Alonso del AC, Iqbal K, Grundke-Iqbal I (1993) Microtubule associated protein tau: abnormal phohphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268:24374–24384
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259:5301–5305
Alonso del AC, Zaidi T, Grundke-Iqbal I, Iqbal K (1994) Role of abnormally phosphorylated tau in the breakdown of microtubule in Alzheimer disease. Proc Natl Acad Sci USA 19:5562–5566
Alonso del AC, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K (2004) Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 279:34878–34881
Guillemin GJ, Smythe G, Takikawa O, Brew BJ (2005) Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49:15–23
Bialojan C, Takai A (1988) Inhibitory effect of a marine spongetoxin, okadaic acid, on protein phosphatases. Biochem J 256:283–290
Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K (1995) Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 65:732–738
Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61:921–927
Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22:1942–1950
Janssens V, Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J 353:417–439
Lechward K, Awotunde OS, Swiatek W, Muszynska G (2001) Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim Pol 48(4):921–933
Turowski P, Favre B, Campbell KS, Lamb NJC, Hemmings BA (1997) Modulation of the enzymatic properties of protein phosphatase 2A catalytic subunit by the recombinant 65-kDa regulatory subunit PR65a. Eur J Biochem 248:200–208
Agostinis P, Goris J, Waelkens E, Pinna LA, Marchiori F, Merlevede W (1987) Dephosphorylation of phosphoproteins and synthetic phosphopeptides Study of the specifcity of the polycation-stimulated and MgATP-dependent phosphorylase phosphatases. J Biol Chem 262:1060–1064
Li N, Yu ZL, Wang L, Zheng YT, Jia JX, Wang Q, Zhu MJ, Liu XL, Xia X, Li WJ (2010) Increased tau phosphorylation and beta amyloid in the hippocampus of mouse pups by early life lead exposure. Acta Biol Hung 61(2):123–134
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder L (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Tomlinson BE, Blessed G, Roth M (1970) Observations on the brains of demented old people. J Neurol Sci 11:205–242
Tolnay M, Probst A (1999) Review: tau protein pathology in Alzheimer’s disease and related disorders. Neuropathol Appl Neurobiol 25:171–187
Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B (1987) Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol (Berl) 74:209–225
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Kosmidis S, Grammenoudi S, Papanikolopoulou K, Skoulakis EM (2010) Differential effects of Tau on the integrity and function of neurons essential for learning in Drosophila. J Neurosci 30(2):464–477
Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, Joels M, van Leuven F, Lucassen PJ (2006) Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci 26(13):3514–3523
Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C (2007) Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci 27(14):3650–3662
Krigman MR, Hogan EL (1974) Effect of lead intoxication on the postnatal growth of the rat nervous system. Environ Health Perspect 7:187–199
Goyer RA (1990) Transplacental transport of lead. Environ Health Perspect 89:101–115
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rahman, A., Brew, B.J. & Guillemin, G.J. Lead Dysregulates Serine/Threonine Protein Phosphatases in Human Neurons. Neurochem Res 36, 195–204 (2011). https://doi.org/10.1007/s11064-010-0300-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0300-6