Abstract
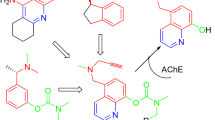
chelators can modulate β-amyloid accumulation, protect against tau hyperphosphorylation, and block metal-related oxidative stress, and thereby hold considerable promise as effective anti-AD drugs. At present, a growing interest is focusing on increasing the efficacy and targeting of chelators through drug design. To this end, we have developed a new class of multifunctional prochelators from three FDA- approved drugs rasagiline, rivastigmine, and donepezil or tacrine. HLA20 A was designed by merging the important pharmacophores of rasagiline, rivastigmine, and donepezil into our newly developed multifunctional chelator HLA20. M30D was constructed using the key pharmacophoric moieties from rasagiline, rivastigmine, and tacrine. Experiments showed that both compounds possess potent anti-acetylcholinesterase (AChE) activity in vitro with weak inhibition of butyrylcholinesterase (BuChE), and without significant metal-binding activity. M30D was found also to be a highly potent MAO A inhibitor with moderate inhibition of MAO B in vitro. Both HLA20 and M30D can be activated by inhibition of AChE to release active chelators HLA20 and M30, respectively. HLA20 and M30 have been shown to be able to modulate amyloid precursor protein regulation and beta-amyloid reduction, suppress oxidative stress, and passivate excess metal ions (Fe, Cu, and Zn). Compared with the activated chelator HLA20 or M30, both HLA20A and M30D exhibited lower cytotoxicity in SH-SY5Y neuroblastoma cells, substantiating the prochelator strategy for minimizing toxicity associated with poor targeted chelators.






Similar content being viewed by others
References
Biran Y, Masters CL, Barnham KJ et al (2009) Pharmacotherapeutic targets in Alzheimer’s disease. J Cell Mol Med 13:61–86
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430:631–639
Barten DM, Albright CF (2008) Therapeutic strategies for Alzheimer’s disease. Mol Neurobiol 37:171–186
Lovell MA, Robertson JD, Teesdale WJ et al (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158:47–52
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol 12:222–228
Bandyopadhyay S, Huang X, Cho H et al (2006) Metal specificity of an iron-responsive element in Alzheimer’s APP mRNA 5’untranslated region, tolerance of SH-SY5Y and H4 neural cells to desferrioxamine, clioquinol, VK-28, and a piperazine chelator. J Neural Transm Suppl 71:237–247
Rogers JT, Randall JD, Cahill CM et al (2002) An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem 277:45518–45528
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Price KA, Crouch PJ, White AR (2007) Therapeutic treatment of Alzheimer’s disease using metal complexing agents. Recent Pat CNS Drug Discov 2:180–187
Adlard PA, Cherny RA, Finkelstein DI et al (2008) Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 59:43–55
Crapper McLachlan DR, Dalton AJ, Kruck TP et al (1991) Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 337:1304–1308
Kaur D, Yantiri F, Rajagopalan S et al (2003) Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron 37:899–909
Arasaki K, Nakanishi T (1989) Selective neurotoxicity of clioquinol on the function of the posterior column nuclei. Neurosci Lett 107:85–88
Porter JB, Huehns ER (1989) The toxic effects of desferrioxamine. Baillieres Clin Haematol 2:459–474
Zheng H, Youdim MB, Fridkin M (2009) Site-activated multifunctional chelator with acetylcholinesterase and neuroprotective-neurorestorative moieties for Alzheimer’s therapy. J Med Chem 52:4095–4098
Chen JJ, Swope DM, Dashtipour K (2007) Comprehensive review of rasagiline, a second-generation monoamine oxidase inhibitor, for the treatment of Parkinson’s disease. Clin Ther 29:1825–1849
Munoz-Torrero D (2008) Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr Med Chem 15:2433–2455
Zhang HY, Yan H, Tang XC (2008) Non-cholinergic effects of huperzine A: beyond inhibition of acetylcholinesterase. Cell Mol Neurobiol 28:173–183
Amit T, Avramovich-Tirosh Y, Youdim MB et al (2008) Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J 22:1296–1305
Zheng H, Gal S, Weiner LM et al (2005) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J Neurochem 95:68–78
Zheng H, Weiner LM, Bar-Am O et al (2005) Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. Bioorg Med Chem 13:773–783
Youdim MB, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Saura J, Luque JM, Cesura AM et al (1994) Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 62:15–30
Reinikainen KJ, Soininen H, Riekkinen PJ (1990) Neurotransmitter changes in Alzheimer’s disease: implications to diagnostics and therapy. J Neurosci Res 27:576–586
Yogev-Falach M, Bar-Am O, Amit T et al (2006) A multifunctional, neuroprotective drug, ladostigil (TV3326), regulates holo-APP translation and processing. FASEB J 20:2177–2179
Zheng H, Youdim MB, Fridkin M (2010) Site-activated chelators targeting acetylcholinesterase and monoamine oxidase for Alzheimer’s therapy. ACS Chem Biol 5:603–610
Groner E, Ashani Y, Schorer-Apelbaum D et al (2007) The kinetics of inhibition of human acetylcholinesterase and butyrylcholinesterase by two series of novel carbamates. Mol Pharmacol 71:1610–1617
Maurer T, Fung HL (2000) Comparison of methods for analyzing kinetic data from mechanism-based enzyme inactivation: application to nitric oxide synthase. AAPS PharmSci 2:E8
Kitz R, Wilson IB (1962) Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem 237:3245–3249
Gal S, Abassi ZA, Youdim MB (2010) Limited potentiation of blood pressure in response to oral tyramine by the Anti-Parkinson Brain Selective Multifunctional Monoamine Oxidase-AB Inhibitor, M30. Neurotox Res 18(2):143–150
Gal S, Zheng H, Fridkin M et al (2005) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J Neurochem 95:79–88
Avramovich-Tirosh Y, Amit T, Bar-Am O et al (2007) Therapeutic targets and potential of the novel brain- permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30, for the treatment of Alzheimer’s disease. J Neurochem 100:490–502
Avramovich-Tirosh Y, Reznichenko L, Mit T et al (2007) Neurorescue activity, APP regulation and amyloid-beta peptide reduction by novel multi-functional brain permeable iron- chelating- antioxidants, M-30 and green tea polyphenol, EGCG. Curr Alzheimer Res 4:403–411
Bush AI (2008) Drug development based on the metals hypothesis of Alzheimer’s disease. J Alzheimers Dis 15:223–240
Cavalli A, Bolognesi ML, Minarini A et al (2008) Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem 51:347–372
Schugar H, Green DE, Bowen ML et al (2007) Combating Alzheimer’s disease with multifunctional molecules designed for metal passivation. Angew Chem Int Ed Engl 46:1716–1718
Liu G, Men P, Kudo W et al (2009) Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer disease. Neurosci Lett 455:187–190
Charkoudian LK, Pham DM, Franz KJ (2006) A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. J Am Chem Soc 128:12424–12425
Folk DS, Franz KJ (2010) A prochelator activated by beta-secretase inhibits Abeta aggregation and suppresses copper-induced reactive oxygen species formation. J Am Chem Soc 132:4994–4995
Di Stefano A, Sozio P, Cocco A et al (2006) L-dopa- and dopamine-(R)-alpha-lipoic acid conjugates as multifunctional codrugs with antioxidant properties. J Med Chem 49:1486–1493
Rodriguez-Rodriguez C, Sanchez de Groot N, Rimola A et al (2009) Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J Am Chem Soc 131:1436–1451
Hindo SS, Mancino AM, Braymer JJ et al (2009) Small molecule modulators of copper-induced Abeta aggregation. J Am Chem Soc 131:16663–16665
Acknowledgments
We thank the Alzheimer Association (USA), Alzheimer Drug Discovery Foundation (New York, USA), Technion-Research and Development and the Weizmann Institute for generous support of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
I do not recall exactly when I first met Abel, but I think it was in Budapest many years ego. What I recall was the fact he was so complimentary to my work as young neurochemist and his encouragement throughout the years I have known him. I was very much moved and honored when decided to dedicate one volume of Neurochemical Research for my contributions and he wrote the most flattering preface. Besides his tremendous scientific contributions, it is his Hungarian charm and gentleness that I so admire, including his unflenching friendship. This has made him many friends and admires from all the world. Moussa B.H. Youdim.
Special Issue: In Honor of Dr. Abel Lajtha.
Rights and permissions
About this article
Cite this article
Zheng, H., Fridkin, M. & Youdim, M.B.H. Site-Activated Chelators Derived from Anti-Parkinson Drug Rasagiline as a Potential Safer and More Effective Approach to the Treatment of Alzheimer’s Disease. Neurochem Res 35, 2117–2123 (2010). https://doi.org/10.1007/s11064-010-0293-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0293-1