Abstract
The present work was conducted to investigate the antioxidant activity and neuroprotective effects of Tripterygium regelii extract (TRE) on H2O2-induced apoptosis in human dopaminergic cells, SH-SY5Y. TRE possessed considerable amounts of phenolics (282.73 mg tannic acid equivalents/g of extract) and flavonoids (101.43 mg naringin equivalents/g of extract). IC50 values for reducing power and DPPH radical scavenging activity were 52.51 and 47.83 μg, respectively. The H2O2 scavenging capacity of TRE was found to be 57.68 μM × μg−1 min−1. By examining the effects of TRE on SH-SY5Y cells injured by H2O2, we found that after incubation of cells with TRE prior to H2O2 exposure, the H2O2 induced cytotoxicity was significantly reversed and the apoptotic features such as change in cellular morphology, nuclear condensation and DNA fragmentation was inhibited. Moreover, TRE was very effective attenuating the disruption of mitochondrial membrane potential and apoptotic cell death induced by H2O2. TRE extract effectively suppressed the up-regulation of Bax, Caspase-3 and -9, and down-regulation of Bcl-2. Moreover, TRE pretreatment evidently increased the tyrosine hydroxylase (TH) and brain-derived neurotrophic factor (BDNF) in SH-SY5Y cells. These findings demonstrate that TRE protects SH-SY5Y cells against H2O2-induced injury and antioxidant properties may account for its neuroprotective actions and suggest that TRE might potentially serve as an agent for prevention of neurodegenerative disease associated with oxidative stress.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, affecting over six million people worldwide [1]. Pathologically, PD is characterized by the marked degeneration of dopaminergic (DArgic) neurons in the substantia nigra pars compacta. Although the mechanism underlying selective degeneration of DArgic neurons is not known completely, the data indicate that oxidative stress has been reported to play important roles [2, 3]. Accumulating evidences show that oxidative stress is one of the important pathways leading to neuronal cell death in PD. Although the source of increased oxidative stress is not completely known, environmental factor, excitotoxin, dopamine homeostasis and others have gained more attention [4]. Oxidative stress may induce mitochondrial dysfunction, genetic mutation and protein aggregation, and ultimately cause cell death [5]. Oxidative damage mediated by reactive oxygen species (ROS) which can be generated following cell lysis, oxidative burst, or the presence of an excess of free transition metals, can attack protein, deoxyneuclic acid, and lipid membranes, thereby disrupting cellular function and integrity [6, 7]. Among a great variety of ROS, hydrogen peroxide (H2O2) is known to play a pivotal role because it is generated from all sources of oxidative stress and can diffuse freely in and out of cells and tissues [8]. In the brain several antioxidant molecules such as ascorbate, superoxide dismutase, and glutathione peroxidase can remove ROS and protect against oxidative stress. Therefore, therapeutic strategies aimed at preventing or delaying ROS induced apoptosis might be reasonable choice for treatment of neurological diseases.
Recently, attention has been focused on searching for natural substances with neuroprotective potential that can scavenge free radicals and protect cell from oxidative damage. Tripterygium regelii (T. regelii) Sprague et Takeda is one of the most common traditional medicinal herb, native to Korea and Japan. The herb T. regelii has been used in traditional Korean medicine for centuries [9] and has been reported to have various pharmacological properties including anti-cancer and anti-inflammatory effects [10–12]. Diterpene-quinonones and triterpenoids were reported from T. regelii and have been shown that triptoquinone A and B inhibited growth of P-3888 leukemia cells in vitro [13, 14]. Over 300 compounds have been identified in the genus Tripterygium and many of them have been evaluated for biological activity [15]. Tripterygium willfordii Hook.F. is among the most studied plant of the genus Tripterygium. Nevertheless, no information is available regarding the effect of T. regelii extract against the pathogenesis of PD. Therefore, this study was designed to explore the antioxidant activity of T. regelii extract and evaluate its neuroprotective effects against H2O2-induced DArgic neuronal damage.
Materials and Methods
Materials
Dulbecco’s Modified Eagle Medium:Nutrients Mix F-12 (1:1, DMEM/F-12), Fetal Bovine Serum (FBS), penicillin and streptomycin were obtained from Gibco BRL (Gaithersburg MD, USA). 1, 1–diphenyl-2-picryl hydrazyl (DPPH), butylated hydroxytoluene (BHT) and ascorbic acid were obtained from Sigma–Aldrich (St. Louis, MO, USA). Hydrogen peroxide (H2O2), DMSO, 4′, 6-diamidino-2-phenylindole (DAPI), Propidium iodide (PI) and Rhodamine 123 were obtained from Sigma–Aldrich (St. Louis, MO, USA). Moloney-murine leukemia virus ribonuclease (M-MLV), oligo dT (deoxythymidine) primer, dNTPs (deoxynucleic acid triphosphate), Taq polymerase, specific primers (for TH, BDNF, and Actin) and 100 bp DNA ladder were purchased from the BioNEER Co. (Korea). Anti-TH antibody was purchased from Affinity BioReagents, Inc. (Golden, CO, USA). Anti-BDNF, Caspase-3, Caspase-9, Bcl-2, and Bax, antibodies were obtained from SANTA CRUZ (Santa cruz, CA, USA). Anti-actin antibody was purchased from Biomeda crop (Foster City, CA, USA). WEST-ZOL plus was obtained from INTRON biotech (Seongnam, Korea). Bicinchoninic acid protein assay kit was obtained from Pierce (Rockford, IL, USA). Protein inhibitor cocktail was obtained from Calbiochem (Darmstadt, Germany). Cytotoxicity Detection Kit (LDH assay) was purchased from Roche Applied Science (Rotkreuz, Switzerland). DeadEnd™ Fluorometric TUNEL System was purchased from Promega coporation (Madison, WI, USA).
Preparation of Tripterygium regelii Extract
T. regelii was collected from the Jiri-mountain, Jeollanam-do, Republic of Korea in the month of April and was authenticated by Professor Myung-Kon Kim, Department of Bio-food technology, Chonbuk National University, South Korea. A voucher specimen was deposited at Chonbuk National University. Two hundred gram of fresh T. regelii leaves were extracted with 2l of methanol at room temperature for 8 week. The extract was filtered through Whatman No. 1 filter paper, and concentrated using a rotary vacuum evaporator. The concentrate was freeze-dried and its yield was 1.4%. The final extract was a dark brown powder. This powder was then dissolved in phosphate buffered saline (PBS) and filtered through 0.2 μm membrane filter (Millipore, Bedford, MA, USA) and stored at 4°C. It was designated as TRE.
Determination of Total Flavonoid Content
The total flavonoid content was determined according to the method of Moreno et al., [16] with some modifications. Briefly, 100 μl of TRE was diluted with 80% aqueous ethanol (900 μl). An aliquot of 100 μl was added to eppendorf tubes containing 20 μl of 10% aluminum nitrate, 20 μl of 1 M potassium acetate and 860 μl of 80% ethanol. After 40 min at room temperature, the absorbance was determined spectrophotometrically at 415 nm. Total flavonoid concentration was calculated using naringin as standard.
Determination of Total Phenolic Content
The total phenolic content of each extract was estimated by a colorimetric assay based on procedures described by Singleton and Rossi [17] with some modifications. Briefly, a stock solution of extract was prepared by dissolving 1 mg of TRE in 1 ml of pure water. 25 μl of sample from stock solution was mixed with 500 μl Folin-Ciocalteu reagent. After 5 min, 500 μl of Na2CO3 (7.5% w/v) solution was added and the mixture was allowed to stand for 90 min with intermittent shaking. After, 90 min, the absorbance was measured at 725 nm. The final results were expressed as mg of tannic acid equivalents (TAE) per g of samples [18].
DPPH Radical Scavenging Activity
Free radical-scavenging activity of the extracts was estimated according to the method reported by Blois [19] with some modifications. In brief, samples were dissolved in absolute methanol and then centrifuged to remove insoluble materials. Ascorbic acid, quercetin and BHT were used as standards. 90 μl of 0.3 mM DPPH in methanol was mixed with 10 μl of TRE solutions with different concentrations (10, 50, 100, 300, and 500 μg/ml). The 96-well plate was allowed to stand at room temperature for 30 min. A control was prepared as described above without TRE, or standards. Methanol was used for baseline correction. The changes in the absorbance of all the samples and standards were measured at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Radical scavenging activity was calculated using the following formula:
Radical scavenging activity (%) = (ODcontrol−ODsamples)/ODcontrol × 100
Hydrogen Peroxide Scavenging Activity
Hydrogen peroxide (H2O2) scavenging activity was carried out according to the method of Aebi [20] with some modifications. Briefly, 990 μl of 75 mM phosphate buffer (pH 7.0) containing 25 mM H2O2 and 10 μl of 0.2 mg/ml TRE were mixed together. The mixture was incubated at 37°C for 2 min, and the absorbance was measured at 240 nm. Specific absorption coefficient of H2O2 −0.03408 cm−1mM−1 was used for calculation. Results were determined using the following equation:
H2O2 scavenging activity = decreasing H2O2 (μM)/{sample weight (mg) × reaction time (min)}
Reducing Power
The reducing power of extracts was determined according to the method of Oyaizu [21] with some modification. Briefly, 200 μl of TRE at various concentrations (10, 50, 100, 300, 500 μg/ml) were mixed with 0.2 M phosphate buffer solution (200 μl, pH 6.6) and 1% potassium ferricyanide (200 μl). The mixture was incubated at 50°C for 20 min. A portion (200 μl) of 10% trichloroacetic acid (TCA) was added to the mixture, which was then centrifuged at 12,000×g for 10 min. The upper layer of solution (500 μl) was mixed with deionized water (500 μl) and 0.1% of ferric chloride (100 μl) in eppendorf tube, and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid and quercetin were used as standard.
Cell Culture and Treatments
The human DA neuronal cell line, SH-SY5Y was obtained from ATTC (Rockville MD). Cells were cultured in DMEM/F12 medium (GIBCO, Gaithersburg) supplemented with 10% FBS and penicillin (100 units/ml)-streptomycin (100 μg/ml) at 37°C in 5% CO2. Media were changed every 2 days. To examine possible toxic effects, SH-SY5Y cells were treated with the TRE extract in a concentration ranging from 1.25 to 20 μg/ml for 24 h. Similarly, cells were treated with H2O2 at concentrations ranging from 25 to 400 μM for 24 h. 10 μg/ml of TRE extract, which was non toxic and 100 μM H2O2 was chosen to evaluate the neuroprotective effects by examining cell viability. TRE extract was added 30 min prior to treatment with H2O2. In a single experiment each treatment was performed in triplicate.
Analysis of Cell Viability
Cell viability was determined by the MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay. SH-SY5Y cells were seeded in 96-well plates at a density of 1 × 104 cell/well and incubated for 24 h prior to experimental treatments. The cells were then subjected to the treatments of interest. After 24 h incubation, MTT (0.5 mg/ml) was added to each wall. Following an additional 3 h incubation at 37°C, 100 μl of DMSO was added to dissolve the formazan crystals. The absorbance was then measured at 540 nm using a VERSAmax micro plate reader (Molecular Devices, CA, USA). Wells without cells were used as blanks and were subtracted as background from each sample. Results were expressed as a percentage of control.
Lactate Dehydrogenase Release Assay
Cells dying by apoptosis or necrosis released Lactate Dehydrogenase (LDH) into the supernatant. The amount of LDH in the supernatant was measured with a cytotoxicity detection kit (Roche). Briefly, the cells (1 × 104 cell/well) were seeded in 96 well plates and then treated with H2O2 for 24 h after pre-treated with or without TRE extract for 30 min. For analysis, 100 μl supernatant was extracted from each well and was placed in separate wells of a new 96-well plate, and 100 μl catalyst solutions was added to each well and incubated at 37°C for 30 min. Absorbance was measured at 490 nm using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Total cellular LDH was determined by lysing the cells with 2% Triton X-100 (high control); the assay medium served as a low control and was subtracted from all absorbance measurements; Cytotoxicity (%) = (exp.value − low control)/(high control − low control) × 100
Observations of Morphological Changes
Cells were seeded in 8-well chamber slide and then treated with H2O2 for 24 h after pre-treated with or without TRE extract for 30 min. The cells were washed twice with PBS and then fixed in 1% paraformaldehyde for 15 min. After rinses with PBS, cellular morphology was observed using a phase contrast microscope (Nikon, Eclipse TE 2000-U, Japan) and photographed.
Nuclear Staining for Assessment of Apoptosis
Nuclear morphology was assessed by staining with 4′, 6-diamidino-2-phenylindole (DAPI).
The cells (1 × 103 cells/well) were seeded in 8-well chamber slide and then treated with H2O2 for 24 h after pre-treated with or without TRE extract for 30 min. The cells were washed twice with PBS and then fixed in 1% paraformaldehyde for 15 min. After two rinses with PBS, the cells were stained with DAPI (0.3 μM) for 10 min at 37°C in dark. Slides were washed twice with PBS and examined under fluorescent microscope (Nikon, Eclipse TE 2000-U, Japan) and photographed.
TUNEL Assay
For in situ detection of fragmented DNA, TUNEL assay was performed using DeadEnd™ Fluorometric TUNEL System (Promega coporation, USA). Cells were cultured on 8-well chamber slide at a density of 1 × 103 cells/chamber. After treatment with H2O2 and TRE for 24 h, cells were washed with PBS and fixed by incubation in 4% paraformaldehyde for 20 min at 4°C. The fixed cells were then washed and permeabilized with 0.2% Triton X-100 in PBS for 5 min. After rinses with PBS, the cells were incubated with terminal deoxynucleotidyl transferase recombinant (rTdT)-catalyzed reaction and nucleotide mixture for 60 min at 37°C in dark and then immersed in stop/wash buffer for 15 min at room temperature. The cells were then washed with PBS to remove unincorporated fluorescein-12-dUTP. After washing, cells were incubated in 1 μg/ml propidium iodide (PI) solution for 15 min in dark. The cells were observed with fluorescent microscope (Nikon, Eclipse TE 2000-U, Japan) and photographed.
Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was determined using the fluorescent dye Rhodamine 123. Briefly, the cells were treated with H2O2 for 24 h after pre-treated with or without TRE for 30 min. Cells were washed with PBS and fixed by incubation in 4% paraformaldehyde for 15 min at room temperature. After rinses with PBS, the fixed cells were incubated with 10 μg/ml Rhodamine 123 for 60 min at 37°C. The cells were washed and monitored by fluorescent microscope (Nikon, Eclipse TE 2000-U, Japan) and photographed. The fluorescence intensity was determined using a Spectra Max Gemini EM fluorometer (Molecular Devices, Sunnyvale, CA, USA) at 490 nm excitation and 515 nm emission.
RNA Isolation and Reverse Transcription
Total RNA was extracted using TRIzol reagent (Invitrogen, Life Technologies, USA) following the protocol provided by the company. Two micrograms of total RNA samples were reverse transcribed for each sample to be analyzed by incubation with a reverse transcription mixture containing the following constituents: 10 pmol oligo (dT) primer, 1× PCR buffer, 0.1 M DTT, 10 mM dNTPs, 20 units of RNase inhibitor, and 200 units of M-MLV. The reaction mixture was incubated for 60 min at 42°C, followed by 5 min at 70°C to inactivate the Reverse transcription (RT) enzyme. The quality of cDNA was verified by PCR amplification of β-actin.
Polymerase Chain Reaction (PCR) and the Analysis of PCR Products
The cDNA in the RT product was amplified using Taq DNA polymerase. A PCR reaction was performed in 20 μl of the total volume using 10 pmol of the following primers-TH, F: 5′- GAG GAG AAG GAG GGG AAG-3′ R: 5′- TCC AAG TCC AGG TCA GGG TC-3′; BDNF, F: 5′- GAT GAC CAT CCT TTT CCT TAC TAT GG-3′ R: 5′- CTA TCT TCC CCT TTT AAT GGT CAA T-3′; β- actin, F: 5′- CCT CTA TGC CAA CAC AGT-3′ R:5′- AGC CAC CAA TCC ACA CAG-3′. The cDNA was amplified under the following reaction conditions: denaturation at 94°C for 30 s, annealing at 57°C for 45 s for TH, at 55.8°C for BDNF and at 56°C for β-actin, polymerization at 72°C for 30 s. The cyclic process was performed 35 times for TH, BDNF and 30 times for β-actin. The PCR products were analyzed on 1.2% agarose gel and visualized by EtBr. The stained intensity of individual bands was evaluated by Gel Quant software (DNR Bio-Imaging Systems Ltd.).
Immunoblotting
After treatment, cells were washed once with PBS and then lysed using ice-cold RIPA buffer with protease inhibitor cocktail. Cell lysates were centrifuged at 12,000×g for 25 min, and the protein concentrations were determined by the bicinchoninic acid (BCA) method using bovine serum albumin (BSA) as standard. The proteins were separated by 10.5% SDS–PAGE and transferred to polyvinylidine difluoride (PVDF) membrane. The Membrane was blocked with 5% (v/v) nonfat dry milk in Tris-buffered saline with Tween 20 (TBS-T) (10 mM Tris–HCl, 150 mM NaCl, and 0.1% Tween 20, pH 7.5) and incubated with primary antibody for Bcl-2 (1:2000 dilution), Bax (1:1000 dilution), Caspase 3 (1:1000 dilution), Caspase 9 (1:2000 dilution), TH and BDNF (1:1000 dilution), or Actin (1:4000 dilution) for overnight at 4°C. The membrane was washed in TBS-T and incubated for 2 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibody. To reveal the reaction bands, the membrane was reacted with WEST-ZOL (plus) Western blot detection system (Intron Biotechnology, Inc., Korea) and exposed on X-ray film (BioMax MS-1, Eastman Kodak, USA).
Statistical Analysis
The data were expressed as the means ± SD. Statistical significance was assessed with one-way analysis of variance followed by a post- hoc (Bonferroni) test for multiple group comparison. Differences with P value less than 0.05 were considered statistically significant.
Results
Total Phenolic and Flavonoid Content
As one of the most important antioxidant plant components, phenolic compounds are widely investigated in many medicinal plant and vegetables [22]. Although most antioxidant activities from plant sources are derived from phenolic-type compounds [23], these effects do not always correlate with the presence of large quantities of phenolics. Therefore, both sets of data need to be examined together. For this, the extracts were analyzed for total phenolic and flavonoid contents. The amounts of total phenolic and flavonoid contents are shown in Table 1. The concentration of total phenolics in extracts was estimated by the Folin-Ciocalteu procedure which is considered as the best method for total phenolics determination [24]. The total phenolic content in the TRE was 282.73 ± 10.58 mg tannic acid equivalents/g of extract. The total flavonoid content was estimated by the aluminum nitrate colorimetric method and was found to be 101.43 ± 2.60 mg naringin equivalents/g of extract.
Antioxidant Activity
The antioxidant activity of TRE was evaluated by DPPH radical scavenging activity, H2O2 scavenging activity, and its reducing power. DPPH is a stable free radical donor, which has been widely used to test the free radical scavenging effect of natural antioxidants [25]. As shown in Fig. 1a, 52.05 ± 3.61% of DPPH radicals were scavenged when the concentration of TRE was 50 μg/ml, which is, however, higher than BHT (27.48 ± 3.98%) and lower than that of ascorbic acid (92.67 ± 2.40%), a potent antioxidant to scavenge radicals and quercetin (69.30 ± 0.91), a known flavonoid. In addition, the scavenging activity of these extracts increased with the increase in their concentrations from 10 to 500 μg/ml. The average IC50 concentrations for the TRE, quercetin and vitamin C were found to be 47.83 ± 0.26 μg, 41.83 ± 8.94 μg and 3.24 ± 0.15 μg, respectively (Table 1). These results clearly indicated that TRE must be a potent source of compounds that can donate hydrogen atoms to act as an antioxidant.
Fe(III) reduction is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action, and can be strongly correlated with other antioxidant properties [26]. Figure 1b shows the reducing powers of the TRE. Like the DPPH radical scavenging activity, the reducing power of TRE was concentration dependent. The reducing power of TRE was 0.854 ± 0.015 at 100 μg/ml and 1.322 ± 0.028 at 300 μg/ml. However, ascorbic acid showed slightly higher activity with a reducing power of 1.299 ± 0.028 and 1.477 ± 0.026 at 100 and 300 mg/ml, respectively. The IC50 of TRE was 52.51 ± 0.21 μg while that for quercetin and vitamin C were 43.21 ± 0.96 μg, 24.00 ± 0.32 μg, respectively (Table 1). These results demonstrate the electron donor properties of TRE for neutralizing free radicals by forming stable products.
H2O2 is one of the reactive oxygen species. The H2O2 scavenging activity was determined as a measure of the antioxidant activity of TRE. As can be seen from Table 1, TRE had an effective H2O2 scavenging capacity. Scavenging activity of TRE was found to be 57.68 ± 3.61 μM × μg−1 min−1.
These data showed that TRE could be a source of compounds with potent antioxidant activity.
Effects of TRE Against H2O2-Induced Cytotoxicity
Initial experiments were first performed to determine whether H2O2 alone or TRE alone was toxic to human dopaminergic cells, SH-SY5Y. Cells were exposed to various concentrations of H2O2 (25, 50, 100, 200, and 400 μM) for 24 h and cell survival was assessed by MTT assay. As shown in Fig. 2a, H2O2 induced a dose dependent cytotoxicity in SH-SY5Y cells. In the presence of 100 μM H2O2, there is only 58.98% of viable cells as compared to control cells. To evaluate whether TRE influences neuronal cytotoxicity, SH-SY5Y cells were treated with various concentrations of TRE (1.25, 2.5, 5, 10, and 20 μg/ml) for 24 h. These concentrations of TRE did not show any cytotoxicity in SH-SY5Y cells. Consequently, the treatment of 100 μM H2O2 and 10 μg/ml of TRE for 24 h were chosen in the subsequent experiments.
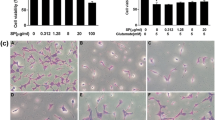
Neuroprotective effects of TRE on H2O2 induced cytotoxicity in SH-SY5Y cells. a Dose-dependent effects of H2O2 on SH-SY5Y cell viability. SH-SY5Y cells were exposed to different concentration of H2O2for 24 h. Cell viability was assessed using MTT assay. b Protective effect of TRE on H2O2-induced cytotoxicity in SH-SY5Y cells. SH-SY5Y cells were pre-treated with 10 μg/ml TRE for 30 min then treated with H2O2 (100 μM) for 24 h and Cell viability was measured using the MTT assay. c Effects of TRE on H2O2 induced morphological alterations in SH-SY5Y cells. Morphological studies were conducted by phase-contrast microscopy. Control cells without any treatment (a), 100 μM H2O2 (b), 10 μg/ml TRE (c), and cells pretreatment with 10 μg/ml TRE for 30 min then treatment with H2O2 (100 μM) (d). (D) SH-SY5Y cells were pre-treated with 10 μg/ml TRE for 30 min then treated with H2O2 (100 μM) for 24 h and Cell toxicity was measured by LDH assay. The data are represented as means ± SD of three independent experiments. Con; untreated control. ** P < 0.01, *** P < 0.001 versus control group; # P < 0.05, ## P < 0.01 versus H2O2 treated group
Next, we attempted to determine the effects of TRE on neuronal protection via on MTT reduction assay. To determine the protective effects of TRE against H2O2-induced loss of cell viability, SH-SY5Y cells were pre-treated with 10 μg/ml TRE extract for 30 min, followed by 100 μM H2O2 for 24 h. As shown in Fig. 2b, H2O2 induced loss of cell viability was significantly attenuated by TRE treatment. The effects of TRE could also be confirmed by the morphological observation (Fig. 2c). Morphological changes were observed in SH-SY5Y cells when treated with 100 μM H2O2 for 24 h (Fig. 2c, b). In this case, the survival cells showed a reduction of cytoplasm and diminution of tack on the plate, in contrast to the typical morphology of cells presented by the untreated control group (Fig. 2c, a). This effect was remarkably reverted when the cells were exposed to H2O2 in the presence of TRE (Fig. 2c, d), indicating that TRE offered protection to the H2O2 induced damage in SH-SY5Y cells.
To further investigate the protective effect of TRE, the release of LDH was measured (Fig. 2d). LDH release is increased as the number of dead cells increases. As shown in Fig. 2d, release of LDH was increased significantly after exposure to 100 μM H2O2, indicating that H2O2 caused cytotoxicity in SH-SY5Y cells. In contrast, TRE-treated cells showed decreased release of LDH compared with H2O2-exposed cell group. The protective effect of TRE on H2O2-induced cytotoxicity determined by LDH assay was similar to that determined by MTT assay. TRE rescued the viability of cells against the neurotoxicity induced by H2O2, suggesting the protective effect of TRE.
Effects of TRE Against H2O2-Induced Apoptosis
Cell body shrinkage, nuclear condensation, and DNA fragmentation are hallmarks of apoptosis. We investigated whether TRE extract prevents apoptosis induced by H2O2 in SH-SY5Y cells. DAPI staining revealed that the control SH-SY5Y cells exhibited normal regular and oval shaped nuclei (Fig. 3a). The nuclear morphology of cells exposed to TRE alone was intact and similar to that of untreated control cells (Fig. 3c). However, H2O2-treated cells (Fig. 3b) exhibited highly condensed and fragmented nuclei morphologies, characteristics of apoptosis. In contrast, TRE pre-treatment inhibited these apoptotic features (Fig. 3d). Further, H2O2 was shown to induce apoptosis by causing DNA strand breaks, as determined by TUNEL assay and PI staining (Fig. 3f, J). Pretreatment of TRE significantly inhibited the H2O2-induced apoptosis (Fig. 3h, L). These results demonstrate that TRE decreased level of cell death, nuclear condensation and DNA fragmentation and indicates that TRE has an anti-apoptotic effect in SH-SY5Y cells.
Inhibition of H2O2 induced apoptosis in SH-SY5Y cells by TRE. Cells were pre-treated with TRE (10 μg/ml, 30 min) then treated with vehicle or H2O2 (100 μM 24 h) Morphological apoptosis was determined by staining with DAPI (A-D), TUNEL (I-L), and PI (E–H). Arrows indicate chromatin condensation, reduced nuclear size and nuclear fragmentation typically observed in apoptotic cells. TRE suppresses these apoptotic features. Each image is representative of three experiments. Pictures were taken using a fluorescent microscope (Nikon, Eclipse TE 2000-U, Japan) and photographed at 100× magnification
Effects of TRE on the H2O2-Induced Reduction of the Mitochondrial Membrane Potential
The diffusion and accumulation of Rodamine- 123 in mitochondria is proportional to the degree of Mitochondrial Membrane Potential (MMP) [27–29]. A collapse of the mitochondrial trans-membrane potential has been linked to several models for apoptosis [30]. To examine if H2O2 induced apoptosis and its rescue by TRE involve on MMP pathway in SH-SY5Y cells, measurement of MMP was carried out using Rodamine 123. The corresponding changes of fluorescence intensity of Rodamine-123 were measured and presented in Fig. 4b. As shown in Fig. 4, when SH-SY5Y cells were exposed to 100 μM H2O2 for 24 h, the mitochondrial membrane potential was significantly decreased, indicating the release of Rodamine-123 from mitochondria into the cytosol. However, the cells pre-incubated with TRE prior to the addition of H2O2, showed a markedly increased in the mitochondrial membrane potential as compared with H2O2-treated cells. These results showed that TRE extract suppressed the H2O2-induced decrease of mitochondrial membrane potential.
Effect of TRE on H2O2-induced decrease of mitochondrial membrane potential. SH-SY5Y cells were pretreated with TRE (10 μg/ml) for 30 min followed by 100 μM H2O2for 24 h. Cells were incubated with Rhodamine 123 and the membrane potential was monitored by fluorescent microscope (Nikon, Eclipse TE 2000-U, Japan) (a). Control cells without any treatment (a), 100 μM H2O2 (b), 10 μg/ml TRE (c), and cells pretreatment with 10 μg/ml TRE for 30 min then treatment with H2O2 (100 μM) (d). The fluorescence intensity was determined using a Spectra Max Gemini EM fluorometer (Molecular Devices, Sunnyvale, CA, USA) at 490 nm excitation and 515 nm emission (b). The reduced fluorescence of Rhodamine 123 was determined as the reduced mitochondrial membrane potential. Results are expressed as mean ± SD of three independent experiments. *** P < 0.001 versus control group; ### P < 0.001 versus H2O2 -treated group
Effects of TRE on Bcl-2 and Bax Protein Expression in H2O2 Treated SH-SY5Y Cells
The Bax to Bcl-2 expression ratio can be used to determine whether a cell has undergone apoptosis. In H2O2-treated cells, the expression of Bcl-2 protein was downregulated whereas the expression of Bax protein was up-regulated (Fig. 5a, c), which resulted in a high Bax to Bcl-2 ratio, indicating that the H2O2-induced apoptosis in SH-SY5Y cells is probably mediated by the mitochondrial pathway. However, pretreatment with TRE attenuated the change in Bax and Bcl-2 that was induced by H2O2, resulting in a decrease in the Bax to Bcl-2 ratio. TRE extract treatment alone maintained the Bax to Bcl-2 ratio compared with control.
TRE prevents H2O2 induced changes in the expression levels of apoptotic proteins. Effects of TRE on Bcl-2 and Bax expression (a, c). Effects of TRE on cleaved caspase-3 and cleaved caspase-9 expression (b, d). SH-SY5Y cells were pretreated with TRE (10 μg/ml) for 30 min followed by 100 μM H2O2 for 24 h. Expression of Bcl-2, Bax, caspase-3, and caspase-9 were assessed by immunoblots and intensity of each band was estimated by densitometric analysis. Actin was used as an internal loading control. Results are expressed as mean ± SD of three independent experiments. * P < 0.05, **P < 0.01, *** P < 0.001 versus control; # P < 0.05, ### P < 0.001 versus H2O2 treated group
Effects of TRE on the H2O2 -Induced Caspase-3, and Caspase-9 Activation
We further investigated the effect of TRE on caspase signaling. Caspase-3 and caspase-9 play an important role in apoptosis, their expression levels were examined by Western blot. As shown in Fig. 5b and d, expression of Caspase-3 and Caspase-9 were markedly increased with the treatment of H2O2. In contrast, TRE pretreatment significantly attenuated the Caspase-3 and Caspase-9 expression in cells treated with H2O2. These results suggest that TRE inhibited downstream apoptotic signaling including the Caspase-3, and Caspase-9.
Effects of TRE on TH and BDNF Expression
As TH plays a key role as a rate-limiting enzyme in the dopamine biosynthesis pathway and BDNF regulates the proliferation, differentiation and survival of dopaminergic neurons, we examined the effect of TRE on TH and BDNF expression. As illustrated in Fig. 6a and b, the mRNA and protein levels of TH and BDNF were dramatically decreased by H2O2-treatment in SH-SY5Y cells. However, treatment with TRE notably induced the expression of these enzymes and maintained the TH and BDNF levels significantly even after H2O2-treatment.
Effects of TRE on TH and BDNF expression. SH-SY5Y cells were pretreated with TRE (10 μg/ml) for 30 min followed by 100 μM H2O2 for 24 h. Expression of the TH and BDNF were detected by PCR (a), and by immunoblot assay (b). Actin was used as an internal loading control. Results are expressed as mean ± SD of three independent experiments. * P < 0.05, compared with untreated control; # P < 0.05, versus H2O2 treated group
Discussion
Progresses in understanding the pathogenesis of neurodegenerative disorders open new avenues for the development of potential neuroprotective therapeutic strategies. Many studies have shown that the oxidative stress is an important mediator of cellular damage in various neurological disorders including PD. Oxidative stress induces ROS such as H2O2 and superoxide anion which results in mitochondrial dysfunction, protein misfolding, genetic mutation and finally cell death [31]. Suppression of ROS by antioxidants might be an effective strategy in inhibiting oxidative stress-induced cell death. Therefore, the use of anti-oxidant agents as a way of neuroprotection could be a potential therapy to slow or ameliorate the progression of neurodegenerative diseases [32]. In this study, we found that TRE exhibited potent DPPH radical scavenging activity, H2O2 scavenging activity and strong reducing power. These results suggest that TRE could be a prominent natural source of strong antioxidant compounds.
Although the composition of TRE extract remains to be elucidated, previously a number of triterpenes, diterpenes, sesquiterpenes and alkaloids as a major compound have been isolated from this plant and their biological activities have been documented [15, 33–39]. Several studies have shown that celastrol, a quinone methide triterpenoid isolated from T. regelii, possesses various biological properties including chemopreventive, antioxidant, neuroprotective and anti-inflammatory actions [33, 37, 38]. Recently, Kim et al. [38] showed that celastrol effectively suppresses the inflammatory responses accompanying the direct inhibition of nitric oxide, prostaglandin E2, cyclooxygenase-2 and inflammatory cytokines in murine macrophages. Moreover, it has been reported that some quinone-methide triterpenes such as celastrol, pristimerin, tingenone, and iguesterin including celastrol isolated from T. regelii exhibited inhibitory effects for SARS-CoV 3CLpro (severe acute respiratory syndrome coronavirus (SARS- CoV) [39]. Natural triterpenoid pristimerin was found to induce mitochondrial cell death through ROS-dependent activation of both Bax and PARP-1 in human cervical cancer cells [40]. In addition, regelin and regelinol, new antitumour ursine type triterpenoids have been reported from this plant [10]. Previous studies have shown the anticancer activities of some sesquiterpenes such as triptofordin F-2, triptogelin A-1, and triptogelin C-1 from T. regelii [41]. Furthermore, diterpene quinoids, triptoquinones A-F isolated from T. regelii have been reported to have anti-inflammatory activities [34, 42]. These studies have demonstrated the potential of TRE to reduce inflammation and autoimmune responses. However, its relationship with neurodegenerative disease is unexplored. In this study we found that TRE could modulate PD-related neurotoxins-induced cell death in human DArgic neurons.
Several studies have shown that H2O2-induces neuronal cell death with more or less necrotic and/or apoptotic characteristics depending on concentrations and exposure time [43–45]. High concentration of H2O2 -induces necrotic forms of cell death [46]. However, moderate concentrations of H2O2 induce DNA cleavage and are associated with morphological evidence of apoptosis [6]. Our results confirmed that treatment of SH-SY5Y cells with H2O2 resulted in a dose dependent viability loss (Fig. 2a). However, pre-treatment with TRE (10 μg/ml) efficiently prevented the loss of cell viability (Fig. 2b, d), which is further confirmed by morphological observations (Fig. 2c). These results indicated that TRE significantly protected SH-SY5Y cells from H2O2-induced cytotoxicity through the inhibition of both apoptotic and necrotic process. Further, in the present study, we showed a protective effect of TRE against H2O2-induced cell death in human DArgic cells, SH-SY5Y. Apoptosis is the process of cell death characterized by cell shrinkage, nuclear condensation, DNA fragmentation and membrane blabbing. These apoptotic features in situ were detected by DAPI, TUNEL and PI staining (Fig. 3). Interestingly, TRE significantly attenuated these features, indicating that TRE may possess an inhibitory effect on H2O2 induced apoptosis.
Apoptosis is mediated through extrinsic pathway by death receptor and intrinsic pathway by mitochondria. Ultimately, these pathways activate caspases, and activated caspases induce cell death. Bcl-2 family consists of two groups; anti-apoptotic group (Bcl-2 and Bcl-xL) and pro-apoptotic group (Bak, Bax, Bid), and they play an important role in mitochondrial related apoptosis pathway. Bcl-2, one of anti-apoptotic factor, residing in the outer mitochondrial membrane inhibits Cytochrome c release [47]. The pro-apoptotic factor Bax, resides in the cytosol. Translocation of Bax to the mitochondrial membrane might lead to loss of mitochondrial membrane potential and an increase in mitochondrial permeability. Increased mitochondrial permeability results in the release of Cytochrome c from the mitochondria [48]. Released Cytochrome c triggers activation of caspase-9 which in turn activates caspase-3, and activated Caspase-3 induces cell death. In this study we examined the modulation of the gene expression of Bcl-2 and Bax after treatments with H2O2. Our data reveal that Bax/Bcl-2 protein ratio is increased following a treatment with H2O2, but it decreases with the administration of TRE prior to H2O2. These results suggest that TRE can indeed diminish H2O2-induced apoptotic cell death. Moreover, we demonstrated that TRE prevented depolarization of mitochondrial membrane potential induced by H2O2 as detected by Rodamine 123 (Fig. 4). In addition, our present findings show that H2O2 induces an activation of caspase-3 and caspase-9, while TRE can prevent these events, supporting a strong anti-apoptotic potential for TRE.
Altogether, our results demonstrate that the H2O2-induced apoptosis in SH-SY5Y cells can be reverted by TRE.
TH is the major rate limiting enzyme of catecholamine biosynthesis in DA and noradrenergic neurons [49]. The expression of TH plays a critical role in survival and differentiation of DArgic neurons. Dramatically reduced numbers of TH-positive neurons was observed in brains of primate and rodent models of Parkinson disease and increased activity of TH in parkinson rats resulted in effective DA production and relief of disease symptoms [50]. It has been reported previously that BDNF is the most important neurotrophic factor for the differentiation and survival of midbrain DA neurons [51, 52]. BDNF can protect DA neurons against neurotoxins in vivo and in vitro [53, 54]. Therefore, the increase of TH and BDNF expression induced by TRE treatment may contribute to their neuroprotective effects.
Although TH and BDNF were upregulated by TRE, the extent of increase differed among them. The degrees of induction were different at the transcriptional as well as translational level. In addition, there was clearly a quantitative difference between TH and BDNF mRNA and protein levels following TRE treatment. This disparity is not surprising since it is well known that the volume of mRNA resulting from gene expression commonly does not lead to equivalent quantities of the target protein [55]. Furthermore, these discrepancies may be due to differences in time course, mode of analysis and most importantly, the experimental conditions.
In conclusion, we found that TRE has DPPH radical scavenging activity, reducing power and H2O2-scavenging activity and neuroprotective effects. The mechanism underlying the protective effects of TRE in H2O2-injured SH-SY5Y cells might be related to the inhibition of apoptotic features such as change in cellular morphology, nuclear condensation and DNA fragmentation, maintenance of MMP stability, increased Bcl-2 activity and decrease of Caspase-3, caspase-9, and Bax activation through mitochondrial-dependent pathway. Moreover, TRE increased the TH and BDNF in SH-SY5Y cells. A probable underlying mechanism of this novel action of TRE may be associated with the presence of flavonoids in the extract, which are a source of antioxidants, since oxidative process are important in the pathogenesis of several disorders including PD. The components with potential antioxidant activity are promising candidates for use as new therapeutic agents against PD. Therefore, TRE may be a highly valuable candidate for the treatment of neurodegenerative disorders associated with oxidative stress.
References
Licker V, Kövari E, Hochstrasser DF et al (2009) Proteomics in human Parkinson’s disease research. J proteomics 73:10–29
Beal MF (2003) Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci 991:120–131
Zhang Y, Dawson VL, Dawson TM (2000) Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol Dis 7:240–250
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Mattson MP, Duan W, Chan SL et al (2002) Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol Aging 23:695–705
Gardner AM, Xu FH, Fady C et al (1997) Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med 22:73–83
Gorman AM, McGowan A, O’Neill C, Cotter T (1996) Oxidative stress and apoptosis in neurodegeneration. J Neurol Sci 139:45–52
Barbouti A, Doulias PT, Nousis L et al (2002) DNA damage and apoptosis in hydrogen peroxide exposed Jurkat cells: bolus addition versus continuous generation of H2O2. Free Radic Biol Med 33:691–702
Ahn DK (1998) Illustrated book of Korean medicinal herbs. Kyo-Hak publishing, Seoul, p 321
Hori H, Pang G-M, Harimaya K et al (1987) Isolation and structure of regelin and regelinol, new antitumor ursine type triterpenoids from Tripterygium regelii. Chem Pharm Bull 35:2125–2128
Lee G-I, Ha JY, Min KR et al (1995) Inhibitory effects of oriental herbal medicines on IL-8 induction in lipopolysaccharide-activated rat macrophages. Planta Med 61:26–30
Byun JH, Kang YS, Kim SS et al (2003) The effect of water extract from Tripterygium regelii on allergy. Kor J Herbology 18:189–199
Shen J, Zhou B (1992a) Studies on diterpene-quinones of Tripterygium regelii Sprague. Chin Chem Lett 3:113–116 (Chem. Abstr. 117:86752)
Shen J, Zhou B (1992b) Triterpenoids of Tripterygium regelii. Zhiwu Xuebao 34:475–479 (Chem. Abstr. 118:187784)
Brinker AM, Ma J, Lipsky PE et al (2007) Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 68:732–766
Moreno MI, Isla MI, Sampietro AR et al (2000) Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol 71:109–114
Singleton VL, Rossi JA Jr (1965) Colorimetric of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Robert YN, Hiroe K, Yotaro K (2008) Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. Seeds. Food Chem 106:760–766
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Oyaizu M (1986) Studies of products browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Djeridane A, Yousfi M, Nadjemi B et al (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 97:654–660
Cai Y, Luo Q, Sun M et al (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
Engelhardt U (2001) Flavonoids-analysis. Crit Rev Food Sci Nutr 41:398–399
Matsukawa R, Dobinsky Z, Kishimoto E et al (1997) A comparison of screening methods for antioxidant activity in seaweeds. J Appl Phycol 9:29–35
Dorman HJD, Peltoketo A, Hiltunen R et al (2003) Characterization of the antioxidant properties of de-odorized aqueous extracts from selected Lamiaceae herbs. Food Chem 83:255–262
Chen LB (1988) Mitochondrial membrane potential in living cells. Annu Rev Cell Biol 4:155–181
Rahn CA, Bombick DW, Doolittle DJ (1991) Assessment of mitochondrial membrane potential as an indicator of cytotoxicity. Fundam Appl Toxicol 16:435–448
Ubl JJ, Chatton JY, Chen S et al (1996) A critical evaluation of in situ measurement of mitochondrial electrical potentials in single hepatocytes. Biochem Biophys Acta 1276:124–132
Prehn JH, Bindokas VP, Jordan J et al (1996) Protective effect of transforming growth factor-beta 1 on beta-amyloid neurotoxicity in rat hippocampal neurons. Mol Pharmacol 49:319–328
Shibata N, Kobayashi M (2008) The role for oxidative stress in neurodegenerative diseases. Brain Nerve 60:157–170
Yuan H, Zheng JC, Liu P et al (2007) Pathogenesis of Parkinson’s disease: oxidative stress, environmental impact factors and inflammatory processes. Neurosci Bull 23:125–130
Jia L (1985) Chemistry and pharmacology and clinical application of the plants of Tripterygium family. Yao Xue Tong Bao 20:1001–1005
Shishido K, Nakano K, Wariishi N et al (1994) Diterpene quinoides from Tripterygium wilfordii var. regelii, which are interleukin-1 inhibitors. Phytochemistry 35:731–737
Duan H, Takaishi Y, Momota H et al (1999) Immunosuppressive diterpenoids from Tripterygium wilfordii. J Nat Prod 62:1522–1525
Lee BW, Seo WD, Gal SW et al (2004) Quinone methide triterpenes from Tripterygium regelii. Agric Chem Biotechnol 47:77–80
Yang H, Chen D, Cui QC et al (2006) Celastrol, a triterpene extracted from the Chinese ‘Thunder of God Vine’,’ is a potent proteasome inhibitor and suppresses human Prostate cancer growth in nude mice. Cancer Res 66:4758–4765
Kim DH, Shin EK, Kim YH et al (2009) Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J Clin Invest 39:819–827
Ryu YB, Park SJ, Kim YM et al (2010) SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg Med Chem Lett 20:1873–1876
Byun JY, Kim MJ, Eum DY et al (2009) Reactive oxygen species-dependent activation of Bax and poly (ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol Pharmacol 76:734–744
Takaishi Y, Ujita K, Tokuda H et al (1992) Inhibitory effects of dihydroagarofuran sesquiterpenes on Epstein–Barr virus activation. Cancer Lett (Shannon, Irel.) 65:19–26
Takaishi Y, Shishido K, Wariishi N et al (1992) Triptoquinone A and B, novel interleukin-1 inhibitors from Tripterygium wilfordii var. regelii. Tetrahedron Lett 33:7177–7180
Cole KK, Perez-Polo JR (2002) Poly (ADP-ribose) polymerase inhibition prevents both apoptotic-like delayed neuronal death and necrosis after H2O2 injury. J Neurochem 82:19–29
Valencia A, Moran J (2004) Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic Biol Med 36:1112–1125
Chen L, Liu L, Yin J et al (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol 41:1284–1295
Lennon SV, Martin SJ, Cotter TG (1991) Dose-dependent induction of apoptosis in human tumor cell lines by widely diverging stimuli. Cell Prolif 24:203–214
Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39:615–647
Chinnaiyan AM, Orth K, O’Rourke K et al (1996) Molecular ordering of the cell death pathway-Bcl-2 and Bcl-x (L) function upstream of ced-3-like apoptotic proteases. J Biol Chem 271:4573–4576
Nagatsu T, Levitt M, Undenfriend S (1964) Tyrosine hydroxylase, the initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Chen JF, Fredduzzi S, Bastia E et al (2003) Adenosine A2A receptors in neuroadaptation to repeated dopaminergic stimulation: implications for the treatment of dyskinesias in Parkinson’s disease. Neurology 61:S74–S81
Knusel B, Winslow JW, Rosenthal A et al (1991) Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin-3. Proc Natl Acad Sci 88:961–965
Seroogy KB, Lundgren KH, Tran TM et al (1994) Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol 342:321–334
Skaper SD, Negro A, Facci L et al (1993) Brain-derived neurotrophic factor selectively rescues mesencephalic dopaminergic neurons from 2, 4, 5-trihydroxyphenylalanine-induced injury. J Neurosci Res 34:478–487
Fawcett JP, Bamji SX, Causing CG et al (1998) Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci 18:2808–2821
Pliego Rivero FB, McCormack WJ, Jauniaux E et al (1999) Forskolin-induced expression of tyrosine hydroxylase in human foetal brain cortex. Brain Res Dev Brain Res 114:201–206
Acknowledgments
This study was supported by technology development program (20080329) of the Ministry of Agriculture and Forestry (ARPC), Republic of Korea; and this study was conducted by research funds from Gwangju University in 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, BS., Sapkota, K., Kim, S. et al. Antioxidant Activity and Protective Effects of Tripterygium regelii Extract on Hydrogen Peroxide-Induced Injury in Human Dopaminergic Cells, SH-SY5Y. Neurochem Res 35, 1269–1280 (2010). https://doi.org/10.1007/s11064-010-0185-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0185-4