Abstract
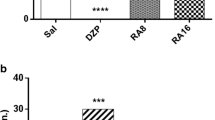
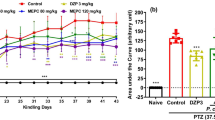
Astragalus mongholicus (AM) is a traditional medicinal herb used as a neuroprotective agent for its anxiolytic, antidepressant, antiamnestic, and antiaggresive effects. However, the mechanisms underlying its anti-convulsant properties are not well studied. In the present study, we examined the anticonvulsant effects on pentylenetetrazol (PTZ)-induced seizures in mice and the possible mechanisms of protection against oxidative damage and mitochondrial dysfunction in vitro. The behavioral studies showed that the root extract of AM had powerful anticonvulsant effects against seizures induced by PTZ and the biochemical studies showed that root extract of AM inhibited PTZ-induced increase in lipid peroxidation, protein oxidation and reactive oxygen species, and enhanced mitochondrial function. Electron spin resonance spectroscopy studies demonstrated that the extracts from the root and aerial parts of AM possess potent effects on scavenging hydroxyl and lipid free radicals. We found that AM extract significantly protected malondialdehyde-induced oxidative damage by ameliorating activities of the mitochondrial complexes I, II, malate dehydrogenase and mitochondrial membrane potential. These data suggest that the anti-convulsant effects of AM extract may be mediated by its protective actions against oxidative damage and amelioration of mitochondrial dysfunction.






Similar content being viewed by others
References
Molodavkin GM, Voronina TA, Aldarmaa J (2000) Psychotropic effect of the Astragalus mongolicus preparation. Eksp Klin Farmakol 63:12–14
Tohda C, Tamura T, Matsuyama S et al (2006) Promotion of axonal maturation and prevention of memory loss in mice by extracts of Astragalus mongholicus. Br J Pharmacol 149:532–541
Castegna A, Thongboonkerd V, Klein JB et al (2003) Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem 85:1394–1401
Arking R, Buck S, Novoseltev VN et al (2002) Genomic plasticity, energy allocations, and the extended longevity phenotypes of Drosophila. Ageing Res Rev 1:209–228
Yoshida Y, Wang MQ, Liu JN et al (1997) Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol 19:359–370
Yang YZ, Jin PY, Guo Q et al (1990) Effect of Astragulas membranaceus on natural killer cell activity and induction of alpha- and gamma-interferon in patients with Coxsackie B viral myocarditis. Chin Med J (Engl) 103:304–307
Mira L, Fernandez MT, Santos M et al (2002) Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res 36:1199–1208
Tian Z, Xiao PG, Wen J et al (2006) Review of bioactivities of natural cycloartane triterpenoids. Zhongguo Zhong Yao Za Zhi 31:625–629
Patel M (2004) Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med 37:1951–1962
Pan JW, Williamson A, Cavus I et al (2008) Neurometabolism in human epilepsy. Epilepsia 49(Suppl 3):31–41
Kudin AP, Kudina TA, Seyfried J et al (2002) Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur J NeuroSci 15:1105–1114
Kunz WS, Kudin AP, Vielhaber S et al (2000) Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann Neurol 48:766–773
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Patsoukis N, Georgiou CD (2004) Determination of the thiol redox state of organisms: new oxidative stress indicators. Anal Bioanal Chem 378:1783–1792
Bashkatova V, Narkevich V, Vitskova G et al (2003) The influence of anticonvulsant and antioxidant drugs on nitric oxide level and lipid peroxidation in the rat brain during penthylenetetrazole-induced epileptiform model seizures. Prog Neuropsychopharmacol Biol Psychiatry 27:487–492
Liu J, Yokoi I, Doniger S et al (1998) Adrenalectomy causes oxidative damage and monoamine increase in the brain of rats and enhances immobilization stress-induced oxidative damage and neurotransmitter changes. Int J Stress Manag 5:39–56
Fukuyama R, Nakayama A, Nakase T et al (2002) A newly established neuronal rho-0 cell line highly susceptible to oxidative stress accumulates iron and other metals. Relevance to the origin of metal ion deposits in brains with neurodegenerative disorders. J Biol Chem 277:41455–41462
Noda Y, Anzai K, Mori A et al (1997) Hydroxyl and superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochem Mol Biol Int 42:35–44
Gomez-Vargas M, Nishibayashi-Asanuma S, Asanuma M et al (1998) Pergolide scavenges both hydroxyl and nitric oxide free radicals in vitro and inhibits lipid peroxidation in different regions of the rat brain. Brain Res 790:202–208
Krahenbuhl S, Chang M, Brass EP et al (1991) Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem 266:20998–21003
Humphries KM, Szweda LI (1998) Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry 37:15835–15841
Trounce IA, Kim YL, Jun AS et al (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509
Picklo MJ, Amarnath V, McIntyre JO et al (1999) 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J Neurochem 72:1617–1624
Reers M, Smiley ST, Mottola-Hartshorn C et al (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417
Liu J, Kabuto H, Hiramatsu M et al (1991) Effects of Guilingji on brain monoamines and their metabolites in mice. Acta Med Okayama 45:217–222
Luszczki JJ, Wojcik-Cwikla J, Andres MM et al (2005) Pharmacological and behavioral characteristics of interactions between vigabatrin and conventional antiepileptic drugs in pentylenetetrazole-induced seizures in mice: an isobolographic analysis. Neuropsychopharmacology 30:958–973
Kokate TG, Svensson BE, Rogawski MA (1994) Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 270:1223–1229
McCord JM (1995) Superoxide radical: controversies, contradictions, and paradoxes. Proc Soc Exp Biol Med 209:112–117
Maertens P, Dyken P, Graf W et al (1995) Free radicals, anticonvulsants, and the neuronal ceroid-lipofuscinoses. Am J Med Genet 57:225–228
Liu J, Mori A (1999) Stress, aging, and brain oxidative damage. Neurochem Res 24:1479–1497
Kodsi MH, Swerdlow NR (1997) Mitochondrial toxin 3-nitropropionic acid produces startle reflex abnormalities and striatal damage in rats that model some features of Huntington’s disease. Neurosci Lett 231:103–107
Chinopoulos C, Adam-Vizi V (2001) Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: relevance to Parkinson’s disease. J Neurochem 76:302–306
Smeitink J, van den Heuvel L, DiMauro S (2001) The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2:342–352
Nicholls DG, Budd SL (2000) Mitochondria and neuronal survival. Physiol Rev 80:315–360
Frantseva MV, Perez Velazquez JL, Tsoraklidis G et al (2000) Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience 97:431–435
Sharma M, Gupta YK (2003) Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur Neuropsychopharmacol 13:241–247
Butterfield DA (2002) Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res 36:1307–1313
Barichello T, Bonatto F, Agostinho FR et al (2004) Structure-related oxidative damage in rat brain after acute and chronic electroshock. Neurochem Res 29:1749–1753
Schneider Oliveira M, Flavia Furian A, Freire Royes LF et al (2004) Ascorbate modulates pentylenetetrazol-induced convulsions biphasically. Neuroscience 128:721–728
Lafon-Cazal M, Pietri S, Culcasi M et al (1993) NMDA-dependent superoxide production and neurotoxicity. Nature 364:535–537
Riazi K, Honar H, Homayoun H et al (2004) Sex and estrus cycle differences in the modulatory effects of morphine on seizure susceptibility in mice. Epilepsia 45:1035–1042
Baylin SB, Herman JG, Graff JR et al (1998) Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 72:141–196
Jodar L, Takahashi M, Kaneto H (1995) Effects of footshock-, psychological- and forced swimming-stress on the learning and memory processes: involvement of opioidergic pathways. Jpn J Pharmacol 67:143–147
Molodavkin GM, Aldarmaa Z, Voronina TA et al (1998) Behavioral and electrophysiologic analysis of the anxiolytic effect of astragalus mongolian. Biull Eksp Biol Med 125:407–409
Acknowledgments
The authors thank Dr. Edward Sharman for critical reading of this manuscript. This study was supported by Pujiang Talent Award (05PG14104; J. L.) from the Shanghai Science and Technology Committee, Shanghai, China and a scholarship of the Third World Academy of Sciences (J. A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aldarmaa, J., Liu, Z., Long, J. et al. Anti-convulsant Effect and Mechanism of Astragalus mongholicus Extract In Vitro and In Vivo: Protection Against Oxidative Damage and Mitochondrial Dysfunction. Neurochem Res 35, 33–41 (2010). https://doi.org/10.1007/s11064-009-0027-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0027-4