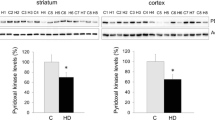
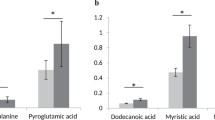
Abstract
3-Nitropropionic acid (3-NP)-induced neurotoxicity can be used as a model for the genetic neurodegenerative disorder Huntington’s disease (HD). A metabolic profiling strategy was adopted to explore the biochemical consequences of 3-NP administered to rats in specific brain regions. 1H NMR spectroscopy was used to characterize the metabolite composition of several brain regions following 3-NP-intoxication. Dose-dependent increases in succinate levels were observed in all neuroanatomical regions, resulting from the 3-NP-induced inhibition of succinate dehydrogenase. Global decreases in taurine and GABA were observed in the majority of brain regions, whereas altered lipid profiles were observed only in the globus pallidus and dorsal striatum. Depleted phosphatidylcholine and elevated glycerol levels, which are indicative of apoptosis, were also observed in the frontal cortex of the 3-NP model. Many of the metabolic anomalies are consistent with those reported in HD. The 3-NP-induced model of HD provides a means of monitoring potential mechanisms of pathology and therapeutic response for drug interventions, which can be efficiently assessed using metabolic profiling strategies.





Similar content being viewed by others
Abbreviations
- NAA:
-
N-Acetylaspartate
- CPMG:
-
Carr–Purcell–Meiboom–Gill
- FID:
-
Free induction decay
- GFAP:
-
Glial fibrillary acid protein
- HD:
-
Huntington’s disease
- H&E:
-
Haematoxylin and eosin
- HR MAS 1H NMR:
-
High-resolution magic angle spinning proton nuclear magnetic resonance spectroscopy
- 3-NP:
-
3-Nitropropionic acid
- SDH:
-
Succinate dehydrogenase
- TCA:
-
Tricarboxylic acid cycle
References
Harris GJ, Pearlson GD, Peyser CE et al (1992) Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington’s disease. Ann Neurol 31:69–75. doi:10.1002/ana.410310113
Myers RH, Leavitt J, Farrer LA et al (1989) Homozygote for Huntington disease. Am J Hum Genet 45:615–618
Tsang TM, Woodman B, McLoughlin GA et al (2006) Metabolic characterization of the R6/2 transgenic mouse model of Huntington’s disease by high-resolution MAS 1H NMR spectroscopy. J Proteome Res 5:483–492. doi:10.1021/pr050244o
Gustine DL (1979) Aliphatic nitrocompounds in crownvetch. Crop Sci 19:197–203
Wilson BJ (1971) Miscellaneous Aspergillus toxins. In: Ciegler A, Kadis S, Ajl SJ (eds) Microbial toxins, vol 6. Academic Press, New York, pp 251–257
Gould DH, Gustine DL (1982) Basal ganglia degeneration, myelin alterations, and enzyme inhibition induced in mice by the plant toxin 3-nitropropanoic acid. Neuropathol Appl Neurobiol 8:377–393. doi:10.1111/j.1365-2990.1982.tb00306.x
Ludolph AC, Seelig M, Ludolph AG et al (1992) ATP deficits and neuronal degeneration induced by 3-nitropropionic acid. Ann NY Acad Sci 648:300–302. doi:10.1111/j.1749-6632.1992.tb24562.x
Ludolph AC, He F, Spencer PS et al (1991) 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can J Neurol Sci 18:492–498
He F, Zhang S, Qian F et al (1995) Delayed dystonia with striatal CT lucencies induced by a mycotoxin (3-nitropropionic acid). Neurology 45:2178–2183
Gould DH, Wilson MP, Hamar DW (1985) Brain enzyme and clinical alterations induced in rats and mice by nitroaliphatic toxicants. Toxicol Lett 27:83–89. doi:10.1016/0378-4274(85)90123-7
Brouillet E, Guyot MC, Mittoux V et al (1998) Partial inhibition of brain succinate dehydrogenase by 3-nitropropionic acid is sufficient to initiate striatal degeneration in rat. J Neurochem 70:794–805
Guyot MC, Hantraye P, Dolan R et al (1997) Quantifiable bradykinesia, gait abnormalities and Huntington’s disease-like striatal lesions in rats chronically treated with 3-nitropropionic acid. Neuroscience 79:45–56. doi:10.1016/S0306-4522(96)00602-1
Riepe M, Ludolph A, Seelig M et al (1994) Increase of ATP levels by glutamate antagonists is unrelated to neuroprotection. NeuroReport 5:2130–2132. doi:10.1097/00001756-199410270-00035
Urbanska EM, Blaszczak P, Saran T et al (1999) AMPA/kainate-related mechanisms contribute to convulsant and proconvulsant effects of 3-nitropropionic acid. Eur J Pharmacol 370:251–256. doi:10.1016/S0014-2999(99)00147-8
Lee WT, Shen YZ, Chang C (2000) Neuroprotective effect of lamotrigine and MK-801 on rat brain lesions induced by 3-nitropropionic acid: evaluation by magnetic resonance imaging and in vivo proton magnetic resonance spectroscopy. Neuroscience 95:89–95. doi:10.1016/S0306-4522(99)00410-8
Beal MF, Brouillet E, Jenkins BG et al (1993) Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci 13:4181–4192
Kim GW, Copin JC, Kawase M et al (2000) Excitotoxicity is required for induction of oxidative stress and apoptosis in mouse striatum by the mitochondrial toxin, 3-nitropropionic acid. J Cereb Blood Flow Metab 20:119–129. doi:10.1097/00004647-200001000-00016
Andrew ER, Eades RG (1959) Removal of dipolar broadening of NMR spectra of solids by spectral rotation. Nature 183:1802. doi:10.1038/1831802a0
Cheng LL, Lean CL, Bogdanova A et al (1996) Enhanced resolution of proton NMR spectra of malignant lymph nodes using magic-angle spinning. Magn Reson Med 36:653–658. doi:10.1002/mrm.1910360502
Millis KK, Maas WE, Cory DG et al (1997) Gradient, high-resolution, magic-angle spinning nuclear magnetic resonance spectroscopy of human adipocyte tissue. Magn Reson Med 38:399–403. doi:10.1002/mrm.1910380307
Waters NJ, Garrod S, Farrant RD et al (2000) High-resolution magic angle spinning (1)H NMR spectroscopy of intact liver and kidney: optimization of sample preparation procedures and biochemical stability of tissue during spectral acquisition. Anal Biochem 282:16–23. doi:10.1006/abio.2000.4574
Cheng LL, Chang IW, Smith BL et al (1998) Evaluating human breast ductal carcinomas with high-resolution magic-angle spinning proton magnetic resonance spectroscopy. J Magn Reson 135:194–202. doi:10.1006/jmre.1998.1578
Humpfer E, Spraul M, Nicholls AW et al (1997) Direct observation of resolved intracellular and extracellular water signals in intact human red blood cells using 1H MAS NMR spectroscopy. Magn Reson Med 38:334–336. doi:10.1002/mrm.1910380224
Tsang TM, Griffin JL, Haselden J et al (2005) Metabolic characterization of distinct neuroanatomical regions in rats by magic angle spinning 1H nuclear magnetic resonance spectroscopy. Magn Reson Med 53:1018–1024. doi:10.1002/mrm.20447
Montgomerie H (1980) In: Drury RAB, Wallington EA (eds) Carleton’s histological technique, 5 edn. Oxford University Press, Oxford
Bancroft JD, Stevens A (1982) In: Bancroft JD, Stevens A (eds) Theory and practice of histological techniques. Churchill Livingstone, Edinburgh
Foxall PJ, Nicholson JK (1998) Nuclear magnetic resonance spectroscopy: a non-invasive probe of kidney metabolism and function. Exp Nephrol 6:409–414. doi:10.1159/000020549
Waters NJ, Holmes E, Waterfield CJ et al (2002) NMR and pattern recognition studies on liver extracts and intact livers from rats treated with alpha-naphthylisothiocyanate. Biochem Pharmacol 64:67–77. doi:10.1016/S0006-2952(02)01016-X
Henke J, Willker W, Engelmann J et al (1996) Combined extraction techniques of tumour cells and lipid/phospholipid assignment by two dimensional NMR spectroscopy. Anticancer Res 16:1417–1427
Wold S, Johannsson E, Cocchi M (1993) 3D-QSAR in drug design, theory, methods, and applications. ESCOM Science
Hoskuldsson A (1996) Prediction methods in science and technology. Thor Publishing, Copenhagen
Wold S, Albano C, Dunn WJ et al (1984) In: Kowalski BR (ed) Mutlivariate data analysis in chemistry. D. Reidel Publishing Company, Dordrecht
Baslow MH (2000) Functions of N-acetyl-l-aspartate and N-acetyl-l-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem 75:453–459. doi:10.1046/j.1471-4159.2000.0750453.x
Hamilton BF, Gould DH (1987) Nature and distribution of brain lesions in rats intoxicated with 3-nitropropionic acid: a type of hypoxic (energy deficient) brain damage. Acta Neuropathol 72:286–297. doi:10.1007/BF00691103
Hamilton BF, Gould DH (1987) Correlation of morphologic brain lesions with physiologic alterations and blood-brain barrier impairment in 3-nitropropionic acid toxicity in rats. Acta Neuropathol 74:67–74. doi:10.1007/BF00688340
Vis JC, Verbeek MM, De Waal RM et al (1999) 3-Nitropropionic acid induces a spectrum of Huntington’s disease-like neuropathology in rat striatum. Neuropathol Appl Neurobiol 25:513–521. doi:10.1046/j.1365-2990.1999.00212.x
Dautry C, Vaufrey F, Brouillet E et al (2000) Early N-acetylaspartate depletion is a marker of neuronal dysfunction in rats and primates chronically treated with the mitochondrial toxin 3-nitropropionic acid. J Cereb Blood Flow Metab 20:789–799. doi:10.1097/00004647-200005000-00005
Martin WR, Wieler M, Hanstock CC (2007) Is brain lactate increased in Huntington’s disease? J Neurol Sci 263:70–74. doi:10.1016/j.jns.2007.05.035
Binienda Z, Frederick DL, Ferguson SA et al (1995) The effects of perinatal hypoxia on the behavioral, neurochemical, and neurohistological toxicity of the metabolic inhibitor 3-nitropropionic acid. Metab Brain Dis 10:269–282. doi:10.1007/BF02109358
Mader I, Roser W, Kappos L et al (2000) Serial proton MR spectroscopy of contrast-enhancing multiple sclerosis plaques: absolute metabolic values over 2 years during a clinical pharmacological study. AJNR Am J Neuroradiol 21:1220–1227
De Stefano NMatthews PM, Arnold DL (1995) Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med 34:721–727. doi:10.1002/mrm.1910340511
Bates TE, Strangward M, Keelan J et al (1996) Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. NeuroReport 7:1397–1400. doi:10.1097/00001756-199605310-00014
Montpetit VJ, Andermann F, Carpenter S et al (1971) Subacute necrotizing encephalomyelopathy A review and a study of two families. Brain 94:1–30. doi:10.1093/brain/94.1.1
Novotny EJ Jr, Singh G, Wallace DC et al (1986) Leber’s disease and dystonia: a mitochondrial disease. Neurology 36:1053–1060
Hassel B, Sonnewald U (1995) Selective inhibition of the tricarboxylic acid cycle of GABAergic neurons with 3-nitropropionic acid in vivo. J Neurochem 65:1184–1191
McGeer EG, McGeer PL (1976) Duplication of biochemical changes of Huntington’s chorea by intrastriatal injections of glutamic and kainic acids. Nature 263:517–519. doi:10.1038/263517a0
Beal MF (1994) Neurochemistry and toxin models in Huntington’s disease. Curr Opin Neurol 7:542–547. doi:10.1097/00019052-199412000-00012
Cicchetti FGould PV, Parent A (1996) Sparing of striatal neurons coexpressing calretinin and substance P (NK1) receptor in Huntington’s disease. Brain Res 730:232–237
Urbanska EM, Blaszczak P, Saran T et al (1998) Mitochondrial toxin 3-nitropropionic acid evokes seizures in mice. Eur J Pharmacol 359:55–58. doi:10.1016/S0014-2999(98)00648-7
Brouillet E, Conde F, Beal MF et al (1999) Replicating Huntington’s disease phenotype in experimental animals. Prog Neurobiol 59:427–468. doi:10.1016/S0301-0082(99)00005-2
Schurr A, Tseng MT, West CA et al (1987) Taurine improves the recovery of neuronal function following cerebral hypoxia: an in vitro study. Life Sci 40:2059–2066. doi:10.1016/0024-3205(87)90098-1
Lehmann A, Hagberg H, Nystrom B et al (1985) In vivo regulation of extracellular taurine and other neuroactive amino acids in the rabbit hippocampus. Prog Clin Biol Res 179:289–311
Law RO (1998) The role of taurine in the regulation of brain cell volume in chronically hyponatraemic rats. Neurochem Int 33:467–472. doi:10.1016/S0197-0186(98)00051-5
Butterworth RF (1996) Taurine in hepatic encephalopathy. Adv Exp Med Biol 403:601–606
Sanchez-Olea R, Pena C, Moran J et al (1993) Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl- transport in cultured astrocytes. Neurosci Lett 156:141–144. doi:10.1016/0304-3940(93)90458-W
Puri BK (2006) Proton and 31-phosphorus neurospectroscopy in the study of membrane phospholipids and fatty acid intervention in schizophrenia, depression, chronic fatigue syndrome (myalgic encephalomyelitis) and dyslexia. Int Rev Psychiatry 18:145–147. doi:10.1080/09540260600581852
Jenkins BG, Koroshetz WJ, Beal MF et al (1993) Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology 43:2689–2695
Anthony MLZhao M, Brindle KM (1999) Inhibition of phosphatidylcholine biosynthesis following induction of apoptosis in HL-60 cells. J Biol Chem 274:19686–19692. doi:10.1074/jbc.274.28.19686
Blankenberg FG, Katsikis PD, Storrs RW et al (1997) Quantitative analysis of apoptotic cell death using proton nuclear magnetic resonance spectroscopy. Blood 89:3778–3786
Blankenberg FG, Storrs RW, Naumovski L et al (1996) Detection of apoptotic cell death by proton nuclear magnetic resonance spectroscopy. Blood 87:1951–1956
Dunlop DSMc Hale DM, Lajtha A (1992) Decreased brain N-acetylaspartate in Huntington’s disease. Brain Res 580:44–48. doi:10.1016/0006-8993(92)90925-Y
Klunk WE, Panchalingam K, Moossy J et al (1992) N-Acetyl-l-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance study. Neurology 42:1578–1585
Meyerhoff DJ, MacKay S, Bachman L et al (1993) Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology 43:509–515
Ebisu T, Rooney WD, Graham SH et al (1994) N-Acetylaspartate as an in vivo marker of neuronal viability in kainate-induced status epilepticus: 1H magnetic resonance spectroscopic imaging. J Cereb Blood Flow Metab 14:373–382
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsang, T.M., Haselden, J.N. & Holmes, E. Metabonomic Characterization of the 3-Nitropropionic Acid Rat Model of Huntington’s Disease. Neurochem Res 34, 1261–1271 (2009). https://doi.org/10.1007/s11064-008-9904-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9904-5