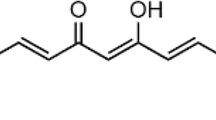
Abstract
Parkinson′s disease (PD) is one of the most important neurodegenerative worldwide disorders. The potential cytoprotective effects of aqueous extract of Valeriana officinalis on rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells were demonstrated. The cytotoxicity, cell viability and analysis of cellular morphology were performed by MTT-tetrazole (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and phase contrast microscopy, respectively. Significant changes in the cellular morphology, and condensation of the cell body could be observed when cells were treated with 300 nM rotenone for 48 h. Three different concentrations of Valeriana officinalis extract were used (0.049, 0.098 and 0.195 mg/mL). These extracts brought about an increase of 7.0 ± 1.3%, 14.5 ± 1.3% and 14.5 ± 3.2% in cell viability. Our results indicated that neuroprotector action of the Valeriana officinalis extract provides support for later studies as they help understanding this drug for the development of cytoprotective various therapies in PD.



Similar content being viewed by others
References
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302(5646):819–822
Spillantini MG, Schmidt ML, Lee VM et al (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840
Greenamyre JT, Hastings TG (2004) Biomedicine: Parkinson’s-divergent causes, convergent mechanisms. Science 304:1120–1122
Cowan WM, Kandel ER (2001) Prospects for neurology and psychiatry. J Am Med Assoc 285:594–600
Eberhardt O, Schulz JB (2003) Apoptotic mechanisms and antiapoptotic therapy in the MPTP model of Parkinson’s disease. Toxicol Lett 4:135–151
Gorell JM, Peterson EL, Rybicki BA et al (2004) Multiple risk factors for Parkinson’s disease. J Neurol Sci 217:169–174
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci 30(5):244–250
Gorell JM, Johnson CC, Rybicki BA et al (1998) The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50:1346–1350
Davies KJ (1995) Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 61:1–31
Greenamyre JT, Sherer TB, Betarbet R et al (2001) Complex I and Parkinson’s disease. IUBMB Life 52:135–141
Li N, Ragheb K, Lawler G et al (2002) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525
Sipos I, Tretter L, Adam-Vizi V (2003) Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem 84:112–118
Seaton TA, Cooper JM, Schapira AH (1997) Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain Res 777:110–118
Betarbet R, Sherer TB, Greenamyre JT (2002) Animal models of Parkinson’s disease. Bioessays 24:308–318
Houghton PJ (1999) The scientific basis for the reputed activity of Valerian. J Pharm Pharmacol 51:505–512
Piccinelli AL, Arana S, Caceres A et al (2004) New lignans from the roots of Valeriana prionophylla with antioxidative and vasorelaxant activities. J Nat Prod 67:1135–1140
Carlini EA (2003) Plants and the central nervous system. Pharmacol Biochem Behav 75:501–512
Hobbs C (1989) Valerian. Herbal Gram 21:19–34
Hori H, Ohmori O, Shinkai T et al (2000) Manganese superoxide dismutase gene polymorphism and schizophrenia: relation to tardive dyskinesia. Neuropsychopharmacology 23:170–177
Molina-Jimenez MF, Sanchez-Reus MI, Benedi J (2003) Effect of fraxetin and myricetin on rotenone-induced cytotoxicity in SH-SY5Y cells: comparison with N-acetylcysteine. Eur J Pharmacol 472:81–87
Cumming JL, Zhong K (2006) Treatments for behavioural disorders in neurodegenerative diseases: drug development strategies. Nat Rev Drug Discov 5:64–74
Carol AN, Linda AA (1996) Herbal medicines—a guide for health-careprofessionals. The Pharmaceutical Press, London
Marder M, Viola H, Wasowskia C et al (2003) 6-Methylapigenin and hesperidin: new Valeriana flavonoids with activity on the CNS. Pharmacology. Biochem Behav 75:537–545
Malva JO, Santos S, Macedo T (2004) Neuroprotective properties of Valeriana officinalis extracts. Neurotox Res 6:131–140
Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 12:203–210
Tan S, Sagara Y, Liu Y et al (1998) The regulation of reactive oxygen species production during programmed cell death. J Cell Biol 141:1423–1432
Sherer TB, Betarbet R, Greenamyre JT (2002) Environment, mitochondria, and Parkinson’s disease. Neuroscientist 8:192–197
Mattson MP (2007) Mitochondrial regulation of neuronal plasticity. Neurochem Res 32:707–715
Valverde G De Andrade D, Madureira de Oliveria D, Barreto G, Bertolino LA, Saraceno E, Capani F, Giraldez LD (2008) Effects of the extract of Anemopaegma mirandum (Catuaba) on Rotenone-induced apoptosis in human neuroblastomas SH-SY5Y cells. Brain Res 1198:188–196
Chung WG, Miranda CL, Maier CS (2007) Epigallocatechin gallate (EGCG) potentiates the cytotoxicity of rotenone in neuroblastoma SH-SY5Y cells. Brain Res 1176:133–142
Betarbet R, Sherer TB, MacKenzie G et al (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Sherer TB, Betarbet R, Stout AK et al (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015
Brown TP, Rumsby PC, Capleton AC et al (2006) Pesticides and Parkinson’s disease—is there a link? Environ. Health Perspect 114:156–164
Dexter DT, Holley AE, Flitter WD (1994) Increase levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord 9:92–97
Jenner P, Olanow CW (1998) Understanding cell death in Parkinson′s disease. Ann Neurol 44:72–84
Beal MF (2002) Oxidatively modified proteins in aging and disease. Free Radic Biol Med 32:797–803
Connolly GP (2001) Cell imaging and morphology. Application to studies of inherited purine metabolic disorders. Pharmacol Ther 90:267–281
Mennini T, Bernasconi P, Bombardelli E et al (1993) In vitro study on the interaction of extracts and pure compounds from Valeriana officinalis roots with GABA, benzodiazepine and barbiturate receptors in rat brain. Fitoterapia 64:291–300
Han D, Zhang QG, Yong-Liu et al (2008) Co-activation of GABA receptors inhibits the JNK3 apoptotic pathway via the disassembly of the GluR6-PSD95-MLK3 signaling module in cerebral ischemic-reperfusion. FEBS Lett 582(9):1298–1306
Newhouse K, Shih-Ling H, Chang SH, Cai B, Wang Y, Xia Z (2004) Rotenone-Induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci 79:137–146
Chen S, Zhang X, Yang D, Du Y, Li L, Li X, Ming M, Le W (2008) D2/D3 receptor agonist ropinirole protects dopaminergic cell line against rotenone-induced apoptosis through inhibition of caspase- and JNK-dependent pathways. FEBS Lett 582(5):603–610
Trauner G, Khom S, Baburin I, Benedek B, Hering S, Kopp B (2008) Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med 74(1):19–24
Wasowski C, Marder M, Viola H et al (2002) Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABA(A) receptors, from Valeriana wallichii. Planta Med 68:934–936
Youdim KA, Spencer JP, Schroeter H et al (2002) Dietary flavonoids as potential neuroprotectants. Biol Chem 383:503–519
Ahlemeyer B, Krieglstein J (2003) Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci 60:1779–1792
Dajas F, Rivera F, Blasina F et al (2003) Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotoxicity Res 5:425–432
Silva BA, Dias ACP, Ferreres F et al (2004) Neuroprotective effect of H. perforatum extracts on beta-amyloid-iduced neurotoxicity. Neurotoxicity Res 6(2):119–130
Areias FM, Rego AC, Oliveira CR et al (2001) Antioxidant effect of flavonoids after ascorbate/Fé(2 +)-induced oxidative stress in cultured retinal cells. Biochem Pharmacol 62:111–118
Prior RL (2003) Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr 78(Suppl. 3):570S–578S
Acknowledgements
This work was supported by grants from Fundação de Amparo a Pesquisa do Estado da Bahia (PRODOC/FAPESB 016/2004) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (FAPESB/CNPq 159/203), Brazil. We wish to thank Prof. Rex Davis for language supervision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveria, D.M., Barreto, G., De Andrade, D.V.G. et al. Cytoprotective Effect of Valeriana officinalis Extract on an In Vitro Experimental Model of Parkinson Disease. Neurochem Res 34, 215–220 (2009). https://doi.org/10.1007/s11064-008-9749-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9749-y