Abstract
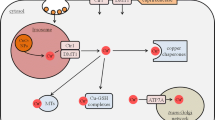
Glutathione (GSH) is one of the major antioxidants in the brain. GSH is secreted by astrocytes and this extracellular GSH is used by neurones to maintain and increase their intracellular GSH levels. For efficient GSH trafficking between astrocytes and neurones, GSH needs to be maintained in the reduced form. In model systems, GSH trafficking has been shown to be essential for neuroprotection against a variety of stress conditions. Previously we and others have shown that GSH and thiols are unstable in cell culture media and are easily oxidised. In the present study it is shown that nanomolar concentrations of copper (II) ions can cause decay of GSH in cell culture media. Increased free or redox active copper has been implicated in a variety of diseases and degradation of extracellular GSH is a possible mechanism by which it exerts its harmful effects. Rat astrocytes, a human astrocytoma cell line and astrocyte-conditioned media, in the absence of cells, are able to retard this copper-catalysed decay of GSH and maintain GSH in its reduced form. The protective effect of astrocytes appears to be a combination of copper removing and antioxidant mechanisms. The importance of these protective mechanisms is discussed with regards to neurodegenerative diseases.






Similar content being viewed by others
Abbreviations
- GSH:
-
Glutathione
- GSSG:
-
Oxidised glutathione
- ETC:
-
Electron transport chain
- CysGly:
-
Cysteinyl-glycine
- γ-GT:
-
γ-Glutamyl transferase
- ApN:
-
Aminopeptidase
- TBARS:
-
Thiobarbituric acid reactive substances
- SOD:
-
Superoxide dismutase
- FBS:
-
Fetal bovine serum
- LDH:
-
Lactate dehydrogenase
- DTPA:
-
Diethylene triamine pentaacetic acid
- OPA:
-
Orthophosphoric acid
- HPLC:
-
High performance liquid chromatography
- BCS:
-
Bathocuproine disulphonate
- CSF:
-
Cerebrospinal fluid
References
Winterbourn CC, Metodiewa D (1994) The reaction of superoxide with reduced glutathione. Arch Biochem Biophys 314:284–290
Sjoberg L, Eriksen TE, Revesz L (1982) The reaction of the hydroxyl radical with glutathione in neutral and alkaline aqueous solution. Radiat Res 89:255–263
Bains JS, Shaw CA (1997) Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev 25:335–358
Sian J, Dexter DT, Lees AJ et al (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Sofic E, Lange KW, Jellinger K et al (1992) Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett 142:128–130
Heales SJ, Davies SE, Bates TE et al (1995) Depletion of brain glutathione is accompanied by impaired mitochondrial function and decreased N-acetyl aspartate concentration. Neurochem Res 20:31–38
Jain A, Martensson J, Stole E et al (1991) Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci USA 88:1913–1917
Bolanos JP, Heales SJ, Peuchen S et al (1996) Nitric oxide-mediated mitochondrial damage: a potential neuroprotective role for glutathione. Free Radic Biol Med 21:995–1001
Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569
Gegg ME, Clark JB, Heales SJ (2005) Co-culture of neurones with glutathione deficient astrocytes leads to increased neuronal susceptibility to nitric oxide and increased glutamate-cysteine ligase activity. Brain Res 1036:1–6
Sagara JI, Miura K, Bannai S (1993) Maintenance of neuronal glutathione by glial cells. J Neurochem 61:1672–1676
Sagara J, Makino N, Bannai S (1996) Glutathione efflux from cultured astrocytes. J Neurochem 66:1876–1881
Hirrlinger J, Schulz JB, Dringen R (2002) Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res 69:318–326
Stewart VC, Stone R, Gegg ME et al (2002) Preservation of extracellular glutathione by an astrocyte derived factor with properties comparable to extracellular superoxide dismutase. J Neurochem 83:984–991
Dringen R, Kranich O, Hamprecht B (1997) The gamma-glutamyl transpeptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture. Neurochem Res 22:727–733
Dringen R, Gutterer JM, Gros C et al (2001) Aminopeptidase N mediates the utilization of the GSH precursor CysGly by cultured neurons. J Neurosci Res 66:1003–1008
Tate SS, Meister A (1974) Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem 249:7593–7602
McIntyre TM, Curthoys NP (1979) Comparison of the hydrolytic and transfer activities of rat renal gamma-glutamyltranspeptidase. J Biol Chem 254:6499–6504
Evans PJ, Tredger JM, Dunne JB et al (1996) Catalytic metal ions and the loss of reduced glutathione from University of Wisconsin preservation solution. Transplantation 62:1046–1049
Hua LL, Halliwell B (2001) Oxidation and generation of hydrogen peroxide by thiol compounds in commonly used cell culture media. Biochem Biophys Res Commun 286:991–994
Kachur AV, Koch CJ, Biaglow JE (1998) Mechanism of copper-catalyzed oxidation of glutathione. Free Radic Res 28:259–269
Wang XF, Cynader MS (2001) Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci 21:3322–3331
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodegenerative disease. Neurobiol Dis 6:221–230
DiDonato M, Sarkar B (1997) Copper transport and its alterations in Menkes and Wilson diseases. Biochim Biophys Acta 1360:3–16
Nagasaka H, Inoue I, Inui A et al (2006) Relationship between oxidative stress and antioxidant systems in the liver of patients with Wilson disease: hepatic manifestation in Wilson disease as a consequence of augmented oxidative stress. Pediatr Res 60:472–477
Lafon-Cazal M, Adjali O, Galeotti N et al (2003) Proteomic analysis of astrocytic secretion in the mouse. Comparison with the cerebrospinal fluid proteome. J Biol Chem 278:24438–24448
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J 245:243–250
White AR, Cappai R (2003) Neurotoxicity from glutathione depletion is dependent on extracellular trace copper. J Neurosci Res 71:889–897
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Protoc 2:223–228
Riederer P, Sofic E, Rausch WD et al (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52:515–520
Rapisarda VA, Volentini SI, Farias RN et al (2002) Quenching of bathocuproine disulfonate fluorescence by Cu(I) as a basis for copper quantification. Anal Biochem 307:105–109
Wee LM, Long LH, Whiteman M et al (2003) Factors affecting the ascorbate- and phenolic-dependent generation of hydrogen peroxide in Dulbecco’s Modified Eagles Medium. Free Radic Res 37:1123–1130
Wilson JX, Peters CE, Sitar SM et al (2000) Glutamate stimulates ascorbate transport by astrocytes. Brain Res 858:61–66
Lonnrot K, Metsa-Ketela T, Molnar G et al (1996) The effect of ascorbate and ubiquinone supplementation on plasma and CSF total antioxidant capacity. Free Radic Biol Med 21:211–217
Dringen R, Hirrlinger J (2003) Glutathione pathways in the brain. Biol Chem 384:505–516
Kapaki E, Zournas C, Kanias G et al (1997) Essential trace element alterations in amyotrophic lateral sclerosis. J Neurol Sci 147:171–175
Jimenez-Jimenez FJ, Molina JA, Aguilar MV et al (1998) Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. J Neural Transm 105:497–505
Stuerenburg HJ (2000) CSF copper concentrations, blood-brain barrier function, and coeruloplasmin synthesis during the treatment of Wilson’s disease. J Neural Transm 107:321–329
Gutteridge JM (1984) Copper-phenanthroline-induced site-specific oxygen-radical damage to DNA. Detection of loosely bound trace copper in biological fluids. Biochem J 218:983–985
Winyard PG, Pall H, Lunec J et al (1987) Non-caeruloplasmin-bound copper (‘phenanthroline copper’) is not detectable in fresh serum or synovial fluid from patients with rheumatoid arthritis. Biochem J 247:245–246
Gaeta A, Hider RC (2005) The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol 146:1041–1059
Pall HS, Williams AC, Blake DR et al (1987) Raised cerebrospinal-fluid copper concentration in Parkinson’s disease. Lancet 2:238–241
Basun H, Forssell LG, Wetterberg L et al (1991) Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J Neural Transm Park Dis Dement Sect 3:231–258
Brown DR (2004) Role of the prion protein in copper turnover in astrocytes. Neurobiol Dis 15:534–543
Brown DR, Qin K, Herms JW et al (1997) The cellular prion protein binds copper in vivo. Nature 390:684–687
Brown DR, Kozlowski H (2004) Biological inorganic and bioinorganic chemistry of neurodegeneration based on prion and Alzheimer diseases. Dalton Trans 1907–1917
Borchardt T, Camakaris J, Cappai R et al (1999) Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion. Biochem J 344(Pt 2):461–467
Terada K, Kawarada Y, Miura N et al (1995) Copper incorporation into ceruloplasmin in rat livers. Biochim Biophys Acta 1270:58–62
Bunton CA (1949) Oxidation of -diketones and -keto-acids by hydrogen peroxide. Nature 163:444
Flohe L, Budde H, Hofmann B (2003) Peroxiredoxins in antioxidant defense and redox regulation. Biofactors 19:3–10
Acknowledgements
This work was supported by the Hospital Savings Association and the Brain Research Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pope, S.A.S., Milton, R. & Heales, S.J.R. Astrocytes Protect Against Copper-Catalysed Loss of Extracellular Glutathione. Neurochem Res 33, 1410–1418 (2008). https://doi.org/10.1007/s11064-008-9602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9602-3