Abstract
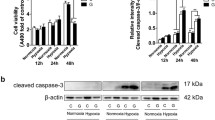
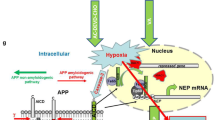
Pathogenesis of Alzheimer’s disease (AD), which is characterised by accumulation of extracellular deposits of β-amyloid peptide (Aβ) in the brain, has recently been linked to vascular disorders such as ischemia and stroke. Aβ is constantly produced in the brain from amyloid precursor protein (APP) through its cleavage by β- and γ-secretases and certain Aβ species are toxic for neurones. The brain has an endogenous mechanism of Aβ removal via proteolytic degradation and the zinc metalloproteinase neprilysin (NEP) is a critical regulator of Aβ concentration. Down-regulation of NEP could predispose to AD. By comparing the effects of hypoxia and oxidative stress on expression and activity of the Aβ-degrading enzyme NEP in human neuroblastoma NB7 cells and rat primary cortical neurones we have demonstrated that hypoxia reduced NEP expression at the protein and mRNA levels as well as its activity. On contrary in astrocytes hypoxia increased NEP mRNA expression.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- NEP:
-
Neprilysin
- ECE:
-
Endothelin converting enzyme
- IDE:
-
Insulin degrading enzyme
- APP:
-
Amyloid precursor protein
- GLUT1:
-
Glucose transporter 1
- PS1:
-
Presenilin 1
References
Atkinson L, Boyle JP, Pearson HA et al (2006) Chronic hypoxia inhibits Na+/Ca2+ exchanger expression in cortical astrocytes. Neuroreport 17:649–652
Di Legge S, Hachinski V (2003) Prospects for prevention and treatment of vascular cognitive impairment. Curr Opin Invest Drugs 4:1082–1087
Kalaria RN (2000) The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging 21:321–330
Camarata PJ, Heros RC, Latchaw RE (1994) “Brain attack”: the rationale for treating stroke as a medical emergency. Neurosurgery 34:144–157
Dolinak D, Smith C, Graham DI (2000) Global hypoxia per se is an unusual cause of axonal injury. Acta Neuropathol (Berl) 100:553–560
Baiden-Amissah K, Joashi U, Blumberg R et al (1998) Expression of amyloid precursor protein (β-APP) in the neonatal brain following hypoxic ischaemic injury. Neuropathol Appl Neurobiol 24:346–352
Webster NJ, Green KN, Peers C et al (2002) Altered processing of amyloid precursor protein in the human neuroblastoma SH-SY5Y by chronic hypoxia. J Neurochem 83:1262–1271
Webster NJ, Green KN, Settle VJ et al (2004) Altered processing of the amyloid precursor protein and decreased expression of ADAM 10 by chronic hypoxia in SH-SY5Y: no role for the stress-activated JNK and p38 signalling pathways. Brain Res Mol Brain Res 130:161–169
Pluta R (2002) Astroglial expression of the β-amyloid in ischemia-reperfusion brain injury. Ann N Y Acad Sci 977:102–108
Nalivaeva NN, Fisk L, Canet Aviles RM et al (2003) Effect of prenatal hypoxia on expression of amyloid precursor protein and metallopeptidases in the rat brain. Lett Peptide Sci 10:455–462
Nalivaeva NN, Fisk L, Kochkina EG et al (2004) Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann N Y Acad Sci 1035:21–33
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–568
Gille JJ, Joenje H (1992) Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res 275:405–414
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Mumford RA, Strauss AW, Powers JC et al (1980) A zinc metalloendopeptidase associated with dog pancreatic membranes. J Biol Chem 255:2227–2230
Jogi A, Ora I, Nilsson H et al (2003) Hypoxia-induced dedifferentiation in neuroblastoma cells. Cancer Lett 197:145–150
Jogi A, Ora I, Nilsson H et al (2002) Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 99:7021–7026
Zhang JZ, Behrooz A, Ismail-Beigi F (1999) Regulation of glucose transport by hypoxia. Am J Kidney Dis 34:189–202
Avramovich-Tirosh Y, Amit T, Bar-Am O et al (2007) Therapeutic targets and potential of the novel brain-permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30, for the treatment of Alzheimer’s disease. J Neurochem 100:490–502 [Epub ahead of print Nov 27, 2006]
Weinreb O, Amit T, Bar-Am O et al (2006) Involvement of multiple survival signal transduction pathways in the neuroprotective, neurorescue and APP processing activity of rasagilinr and its propargyl moiety. J Neuroal Transm Suppl 70:457–465
Cedazo-Minguez A, Bonecchi L, Winblad B et al (1999) Nicergoline stimulates protein kinase C mediated α-secretase processing of the amyloid precursor protein in cultured human neuroblastoma SH-SY5Y cells. Neurochem Int 35:307–315
Fisk L, Nalivaeva NN, Turner AJ (2006) Regulation of endothelin-converting enzyme-1 expression in human neuroblastoma cells. Exp Biol Med (Maywood) 231:1048–1053
Nalivaeva NN, Fisk L, Kochkina EG et al (2006) Different mechanisms of secretion of amyloid-degrading enzymes neprilysin and insulinase by human neuroblastoma cells in culture. J Neurochem 98(Suppl):19
Seeger RC, Siegel SE, Sidell N (1982) Neuroblastoma: clinical perspectives, monoclonal antibodies, and retinoic acid. Ann Intern Med 97:873–884
Ross RA, Spengler BA, Biedler JL (1983) Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst 71:741–747
Shapiro DN, Valentine MB, Rowe ST et al (1993) Detection of N-myc gene amplification by fluorescence in situ hybridization. Diagnostic utility for neuroblastoma. Am J Pathol 142:1339–1346
Rosner H, Vacun G, Rebhan M. (1995) Muscarinic receptor-mediated induction of actin-driven lamellar protrusions in neuroblastoma cell somata and growth cones. Involvement of protein kinase C. Eur J Cell Biol 66:324–334
Carson JA, Turner AJ (2002) β-amyloid catabolism: role for neprilysin (NEP) and other metallopeptidases? J Neurochem 81:1–8
Caccamo A, Oddo S, Sugarman MC et al (2005) Age- and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol Aging 26:645–654
Nalivaeva NN, Fisk L, Kochkina EG et al (2006) Developmental dynamics of amyloid-degrading enzymes in the rat brain under normal and hypoxic conditions. Abstracts of FENS meeting. Vienna, Austria 3:335–336
Breteler MM (2000) Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging 21:153–160
Boado RJ, Pardridge WM (2002) Glucose deprivation and hypoxia increase the expression of the GLUT1 glucose transporter via a specific mRNA cis-acting regulatory element. J Neurochem 80:552–554
Vannucci SJ, Seaman LB, Vannucci RC (1996) Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J Cereb Blood Flow Metab 16:77–81
Hoyer A, Bardenheuer HJ, Martin E et al (2005) Amyloid precursor protein (APP) and its derivatives change after cellular energy depletion. An in vitro-study. J Neural Transm 112:239–253
Smith IF, Boyle JP, Vaughan PF et al (2001) Effects of chronic hypoxia on Ca2+ stores and capacitative Ca2+ entry in human neuroblastoma (SH-SY5Y) cells. J Neurochem 79:877–884
Holmquist L, Jogi A, Pahlman S (2005) Phenotypic persistence after reoxygenation of hypoxic neuroblastoma cells. Int J Cancer 116:218–225
Kumar GK, Runold M, Ghai RD et al (1990) Occurrence of neutral endopeptidase activity in the cat carotid body and its significance in chemoreception. Brain Res 517:341–343
Kumar GK, Kou YR, Overholt JL et al (2000) Involvement of substance P in neutral endopeptidase modulation of carotid body sensory responses to hypoxia. J Appl Physiol 88:195–202
Carpenter TC, Stenmark KR (2001) Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. Am J Physiol Lung Cell Mol Physiol 281:L941–L948
Hara H, Oh-hashi K, Yoneda S et al (2006) Elevated neprilysin activity in vitreous of patients with proliferative diabetic retinopathy. Mol Vis 12:977–982
Oh-hashi K, Nagai T, Tanaka T et al (2005) Determination of hypoxic effect on neprilysin activity in human neuroblastoma SH-SY5Y cells using a novel HPLC method. Biochem Biophys Res Commun 334:380–385
Reznichenko L, Amit T, Zheng H et al (2006) Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer’s disease. J Neurochem 97:527–536
Melzig MF, Janka M (2003) Enhancement of neutral endopeptidase activity in SK-N-SH cells by green tea extract. Phytomedicine 10:494–498
Ayoub S, Melzig MF (2006) Induction of neutral endopeptidase (NEP) activity of SK-N-SH cells by natural compounds from green tea. J Pharm Pharmacol 58:495–501
Golde TE (2006) Disease modifying therapy for AD?. J Neurochem 99:689–707
Acknowledgements
This work was supported by Medical Research Council, UK and The Biochemical Society (Mrs L Fisk).
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to Dr. Moussa Youdim.
Rights and permissions
About this article
Cite this article
Fisk, L., Nalivaeva, N.N., Boyle, J.P. et al. Effects of Hypoxia and Oxidative Stress on Expression of Neprilysin in Human Neuroblastoma Cells and Rat Cortical Neurones and Astrocytes. Neurochem Res 32, 1741–1748 (2007). https://doi.org/10.1007/s11064-007-9349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9349-2