Abstract
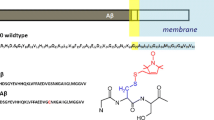
Amyloid beta peptide (Aβ) is a small peptide present in normal cells and aggregated Aβ is the main constituent of the extracellular amyloid plaques found in Alzheimer’s disease (AD) brain. Recent studies suggest that soluble Aβ oligomers are neurotoxic rather than amyloid fibrils found in amyloid plaques. This study using multidimensional NMR spectroscopy and circular dichroism (CD) provides the first direct evidence that alterations in membrane structure can trigger the conversion of soluble α-helical monomeric Aβ into oligomeric Aβ in a β-sheet conformation.
Similar content being viewed by others
References
J. C. Rochet P. T. Lansbury SuffixJr. (2000) ArticleTitleAmyloid fibrillogenesis: Themes and variations Curr. Opin. Struct. Biol. 10 60–68
M. P. Lambert A. K. Barlow B. A. Chromy C. Edwards R. Freed M. Liosatos T. E. Morgan I. Rozovsky B. Trommer K. L. Viola P. Wals C. Zhang C. E. Finch G. A. Krafft W. L. Klein (1998) ArticleTitleDiffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins Proc. Natl. Acad. Sci. USA 94 6448–6453
Y. M. Kuo M. R. Emmerling C. Vigo-Pelfrey T. C. Kasunic J. B. Kirkpatrick G. H. Murdoch M. J. Ball A. E. Roher (1996) ArticleTitleWater-soluble A beta (N-40, N-42) oligomers in normal and Alzheimer disease brains J. Biol. Chem. 271 4077–4081
R. Kayed E. Head J. L. Thompson T. M. McIntire S. C. Milton C. W. Cotman C. G. Glabe (2003) ArticleTitleCommon structure of soluble amyloid oligomers implies common mechanism of pathogenesis Science 300 486–489
K. N. Dahlgren A. M. Manelli W. B. Stine L. K. Baker G. A. Krafft M. J. LaDu (2002) ArticleTitleOligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability J. Biol. Chem. 277 32046–32053
C. M. Rodrigues S. Sola M. A. Brito C. D. Brondino D. Brites J. J. Moura (2001) ArticleTitleAmyloid beta-peptide disrupts mitochondrial membrane lipid and protein structure: Protective role of tauroursodeoxycholate Biochem. Biophys. Res. Commun. 281 468–474
R. P. Mason R. F. Jacob M. F. Walter P. E. Mason N. A. Avdulov S. V. Chochina U. Igbavboa W. G. Wood (1999) ArticleTitleDistribution and fluidizing action of soluble and aggregated amyloid beta-peptide in rat synaptic plasma membranes J. Biol. Chem. 274 18801–18807
R. P. Mason M. W. Trumbore J. W. Pettegrew (1996) ArticleTitleMolecular membrane interactions of a phospholipid metabolite - Implications for Alzheimer’s disease pathophysiology Neurobiol. Alzheimer Dis. 777 368–373
J. W. Pettegrew G. Withers K. Panchalingam J. F. Post (1987) ArticleTitle31P nuclear magnetic resonance (NMR) spectroscopy of brain in aging and Alzheimer’s disease J. Neural. Transm. Suppl. 24 261–268
J. W. Pettegrew S. J. Kopp N. J. Minshew T. Glonek J. M. Feliksik J. P. Tow M. M. Cohen (1987) ArticleTitle31P nuclear magnetic resonance studies of phosphoglyceride metabolism in developing and degenerating brain: Preliminary observations J. Neuropathol Exp. Neurol. 46 419–430
J. W. Pettegrew J. Moossy G. Withers D. McKeag K. Panchalingam (1988) ArticleTitle31P nuclear magnetic resonance study of the brain in Alzheimer’s disease J Neuropathol. Exp. Neurol. 47 235–248
J. W. Pettegrew K. Panchalingam J. Moossy J. Martinez G. Rao F. Boller (1988) ArticleTitleCorrelation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer’s disease Arch. Neurol. 45 1093–1096
J. W. Pettegrew (1989) ArticleTitleMolecular insights into Alzheimer’s disease Ann. N Y Acad. Sci. 563 5–28
J. W. Pettegrew K. Panchalingam R. L. Hamilton R. J. McClure (2001) ArticleTitleBrain membrane phospholipid alterations in Alzheimer’s disease Neurochem. Res. 26 771–782
J. W. Pettegrew K. Panchalingam J. A. Stanley R. J. McClure (2002) ArticleTitleP-31 and H-1 MRSI studies of psychosis in Alzheimer’s disease Am. J. Geriatr. Psychiatry. 10 18–19
S. Dante T. Hauss N. A. Dencher (2002) ArticleTitleBeta-amyloid 25 to 35 is intercalated in anionic and zwitterionic lipid membranes to different extents Biophys. J. 83 2610–2616
M. Bokvist F. Lindstrom A. Watts G. Grobner (2004) ArticleTitleTwo types of Alzheimer’s beta-amyloid (1–40) peptide membrane interactions: Aggregation preventing transmembrane anchoring versus accelerated surface fibril formation J. Mol. Biol. 335 1039–1049
E. Terzi G. Holzemann J. Seelig (1997) ArticleTitleInteraction of Alzheimer beta-amyloid peptide(1–40) with lipid membranes Biochemistry 36 14845–14852
J. W. Pettegrew K. Panchalingam G. Withers D. McKeag S. Strychor (1990) ArticleTitleChanges in brain energy and phospholipid metabolism during development and aging in the Fischer 344 rat J. Neurobiol. Exp. Neuropathol. 49 237–249
J. W. Geddes K. Panchalingam J. N. Keller J. W. Pettegrew (1997) ArticleTitleElevated phosphocholine and phosphatidylcholine following rat entorhinal cortex lesions Neurobiol. Aging 18 305–308
D. P. Tieleman D. Spoel Particlevan der H. J. C. Berendsen (2000) ArticleTitleMolecular dynamics simulations of dodecylphosphocholine micelles at three different aggregate sizes: Micellar structure and chain relaxation J. Phys. Chem. B 104 6380–6388
J. Lauterwein C. Bosch L. R. Brown K. Wuthrich (1979) ArticleTitlePhysicochemical studies of the protein–lipid interactions in melittin-containing micelles Biochim. Biophys. Acta. 566 243–246
B. A. Wallace R. W. Janes A. Orry (2001) ArticleTitleSecondary structure information content on the VUV region of synchrotron radiation circular dichroism (SRCD) spectra Biophys. J. 80 28a
A. Lobley B. A. Wallace (2001) ArticleTitleDichroweb: A website for the analysis of protein secondary structure from circular dichroism spectra. Biophys. J. 80 373a
P. K. Mandal A. Majumdar (2004) ArticleTitleA comprehensive discussion of HSQC and HMQC pulse sequences Concepts Magn. Reson. Part A 20A 1–23
F. Delaglio S. Grzesiek G. W. Vuister G. Zhu J. Pfeifer A. Bax (1995) ArticleTitleNMRPipe: A multidimensional spectral processing system based on UNIX pipes J. Biomol. NMR 6 277–293
D. S. Garrett R. Powers A. M. Gronenborn G. M. Clore (1991) ArticleTitleA common sense approach to peak picking two-, three- and four-dimensional spectra using automatic computer analysis of contour diagrams J. Magn. Reson. 95 214–220
T. D. Goddard D. G. Kneller (1994) SPARKY 3 University of California San Francisco
S. Chandra X. C. Chen J. Rizo R. Jahn T. C. Sudhof (2003) ArticleTitleA broken alpha-helix in folded alpha-synuclein J. Biol. Chem. 278 15313–15318
P. K. Mandal (2002) ArticleTitleComplete NMR spectroscopic assignment of a neuronal transduction protein Monatsh. Chem. 133 205–217
P. K. Mandal J. W. Pettegrew (2004) ArticleTitleAlzheimer’s disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with A beta(1–40) peptide in a membrane mimic environment Neurochem. Res. 29 447–453
K. Pervushin R. Riek G. Wider K. Wuthrich (1997) ArticleTitleAttenuated T-2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution Proc. Natl. Acad. Sci. USA 94 12366–12371
K. Wuthrich (1986) NMR of Proteins and Nucleic Acids John Wiley and Sons Inc. New York
C. D. Schwieters J. J. Kuszewski N. Tjandra G. M. Clore (2003) ArticleTitleThe Xplor-NIH NMR molecular structure determination package J. Magn. Reson. 160 65–73
K. J. Marcinowski H. Shao E. L. Clancy M. G. Zagorski (1998) ArticleTitleSolution structure model of residues 1–28 of the amyloid beta peptide when bound to micelles J. Am. Chem. Soc. 120 11082–11091
H. A. Lashuel D. Hartley B. M. Petre T. Walz P. T. Lansbury SuffixJr. (2002) ArticleTitleNeurodegenerative disease: Amyloid pores from pathogenic mutations. Nature 418 291
D. M. Hartley D. M. Walsh C. P. Ye T. Diehl S. Vasquez P. M. Vassilev D. B. Teplow D. J. Selkoe (1999) ArticleTitleProtofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons J. Neurosci. 19 8876–8884
P. K. Mandal J. W. Pettegrew (2004) ArticleTitleStructure and dynamics studies of ganglioside-Ab peptide in Alzheimer’s disease Biophys. J. 86 340
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, P.K., Pettegrew, J.W. Alzheimer’s Disease: Soluble Oligomeric Aβ(1–40) Peptide in Membrane Mimic Environment from Solution NMR and Circular Dichroism Studies. Neurochem Res 29, 2267–2272 (2004). https://doi.org/10.1007/s11064-004-7035-1
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11064-004-7035-1