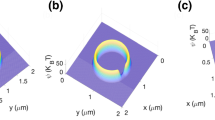
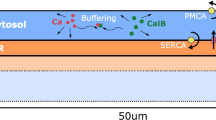
The dependence of intracellular calcium dynamics on geometrical size relations between calcium-exchanging parts of the intracellular space was studied in mathematical models corresponding to a thin fragment of the Purkinje neuron spiny dendrite. The plasma membrane contained ion channels typical of this cell type, including channels that conduct an excitatory synaptic current, and ion pumps. The model equations took into account calcium exchange between the cytosol, extracellular medium, intracellular store (a cistern of the endoplasmic reticulum, ER), endogenous calcium buffers, and an exogenous buffer (fluorescent dye used in the experiments). The ER membrane contained the calcium pump and channels of calcium-dependent and inositol-3-phosphate-dependent calcium release, as well as leakage channels. With the compartment size fixed, the ER cistern diameter was varied so that the proportion of the organelle in the total volume changed from 1 to 36%. Under these conditions, identical synaptic excitation caused similar electrical reactions (calcium spikes) but different concentration responses. Equal increments in the ER diameter led to unequal, more pronounced at thicker diameters, increments of the peak cytosolic concentrations of Са2+ ([Ca2+] i ) and of a Са2+-fluorescent dye complex [CaD], as well as those of the Са2+ concentration in the dendrite ER (characterized by a shift from the basal level, Δ[Ca2+]ER). The changes in [Ca2+] i and [CaD] followed more adequately those in the volume of the organelle-free cytosol, while Δ[Ca2+]ER changes were more similar to those in the ER membrane area. Therefore, the relative occupancy of the intracellular volume by organellar calcium stores and their sizes in a dendritic compartment are important structural factors that essentially modulate the calcium dynamics, and this structural dependence can be adequately reflected in the experiments using fluorophores.
Similar content being viewed by others
References
P. G. Kostyuk and A. Verkhratsky, Calcium Signalling in the Nervous System, Wiley, Chichester (1995).
M. J. Brridge, “Neuronal calcium signalling,” Neuron, 21, No. 1, 13–26 (1998).
Calcium as a Cellular Regulator, E. Carafli and C. Klee (eds.), Oxford Univ. Press, New York (1999).
L. D. Pzzo-Miller, J. A. Connor, and S. B. Andrews, “Microheterogeneity of calcium signalling in dendrites,” J. Physiol., 525, 53–61 (2000).
W. Amada, C. Koch, and P. Adams, “Multiple channels and calcium dynamics,” in: Methods in Neuronal Modeling: From Synapses to Networks, C. Koch and I. Segev (eds.), MIT Press, Cambridge (1989), pp. 137–170.
C. Koch, Biophysics of Computations: Information Processing in Single Neurons, Oxford Univ. Press, New York (1999).
J. Meldolesi, A. Villa, P. Podini et al., “Intracellular Ca2+ stores in neurons. Identification and functional aspects,” J. Physiol., 86, Nos. 1/3, 23–30 (1992).
W. G. Regehr and D. W. Tank, “Dendritic calcium dynamics,” Curr. Opin. Neurobiol., 4, No. 3, 373–382 (1994).
J. Eilrs and A. Konnerth, “Dendritic signal integration,” Curr. Opin. Neurobiol., 7, No. 3, 385–390 (1997).
M. Canepari, K. Vogt, and D. Zecevic, “Combining voltage and calcium imaging from neuronal dendrites,” Cell Mol. Neurobiol., 28, No. 8, 1079–1093 (2008).
E. De Schutter and J. Bower, “An active membrane model of the cerebellar Purkinje cell 1. Simulation of current-clamps in slice,” J. Neurophysiol., 71, 375–400 (1994).
T. Miyasho, H. Takagi, H. Suzuki, et al., “Low-threshold potassium channels and a low-threshold calcium channel regulate Ca2+ spike firing in the dendrites of cerebellar Purkinje neurons: a modeling study,” Brain Res., 891, 106–115 (2001).
P. Achard and E. De Schutter, “Calcium, synaptic plasticity and intrinsic homeostasis in Purkinje neuron models,” Front Comput. Neurosci., 2, 8 (2008).
I. B. Kulagina, S. M. Korogod, G. Horcholle-Bossavit, et al., “The electrodynamics of the dendritic space in Purkinje cells of the cerebellum,” Arch. Ital. Biol., 145, Nos. 3/4, 211–233 (2007).
I. B. Kulagina, “Рhase relationship between calcium and voltage oscillations in different dendrites of Purkinje neuron,” Neurophysiology, 40, Nos. 5/6, 404–416 (2008).
C. A. Ross, J. Meldolesi, T. A. Milner, et al., “Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons,” Nature, 339, No. 6224, 468–470 (1989).
P. Volpe, A. Nori, A. Martini, et al., “Multiple/heterogeneous Ca2+ stores in cerebellum Purkinje neurons,” Comp. Biochem. Physiol. Comp. Physiol., 105, No. 2, 205–211 (1993).
K. Takei, G. A. Mignery, E. Mugnaini, et al., “Inositol 1,4,5-trisphosphate receptor causes formation of ER cisternal stacks in transfected fibroblasts and in cerebellar Purkinje cells,” Neuron, 12, 327–342 (1994).
A. Verkhratsky, “Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons,” Physiol. Rev., 85, 201–279 (2005).
M. Bootman, O. Petersen, and A. Verkhratsky, “The endoplasmic reticulum is a focal point for co-ordination of cellular activity,” Cell Calcium, 32, 231–234 (2002).
D. Hillman and S. Chen, “Plasticity of synaptic size with constancy of total synaptic contact area on Purkinje cells in the cerebellum,” Prog. Clin. Biol. Res., 59A, 229–245 (1981).
J. Takas and J. Hamori, “Developmental dynamics of Purkinje cells and dendritic spines in rat cerebellar cortex,” J. Neurosci. Res., 38, No. 5, 515–530 (1994).
H. Kim, I. Kim, K. J. Lee, et al., “Specific plasticity of parallel fiber/Purkinje cell spine synapses by motor skill learning,” NeuroReport, 13, No. 13, 1607–1610 (2002).
E. Bastianelli, “Distribution of calcium-binding proteins in the cerebellum,” Cerebellum, 2, No. 4, 242–262 (2003).
H. Schmidt, K. M. Stiefel, P. Racay, et al., “Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k,” J. Physiol., 551, No. 1, 13–32 (2003).
H. Schmidt, S. Kunerth, C. Wilms, et al., “Spino-dendritic cross-talk in rodent Purkinje neurons mediated by endogenous Ca2+-binding proteins,” J. Physiol., 581, No. 2, 619–629 (2007).
K. Harris and J. Stevens, “Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics,” J. Neurosci., 8, No. 12, 4455–4469 (1988).
N. T. Carnevale and M. L. Hines, The NEURON Book, Cambridge Univ. Press, Cambridge (2006).
E. De Schutter and P. Smolen, “Calcium dynamics in large neuronal models,” in: Methods in Neuronal Modeling: from Ions to Networks, C. Koch and I. Segev (eds.), MIT Press, Cambridge (1998), pp. 211–250.
S. Dargan, B. Schwaller, and I. Parker, “Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocites by Ca2+-binding proteins,” J. Physiol., 556, No. 2, 447–461 (2004).
J. B. Sorensen, U. Matti, S. H. Wei, et al., “The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis,” Proc. Natl. Acad. Sci. USA, 99, No. 3, 1627–1632 (2002).
D. L. Wokosin, C. M. Loughrey, and G. L. Smith, “Characterization of a range of Fura dyes with two-photon excitation,” Biophys. J., 86, No. 3, 1726–1738 (2004).
E. Neher, “Details of Ca2+ dynamics matter,” J. Physiol., 586, No. 8, 2031 (2008).
N. Volfovsky, H. Parnas, M. Segal, and E. Korkotian, “Geometry of dendritic spines affects calcium dynamics in hippocampal neurons: theory and experiments,” J. Neurophysiol., 82, No. 1, 450–462 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Neirofiziologiya/Neurophysiology, Vol. 41, No. 1, pp. 19–31, January–February, 2009.
Rights and permissions
About this article
Cite this article
Korogod, S.M., Novorodovskaya, T.S. Impact of Geometrical Characteristics of the Organellar Store and Organelle-Free Cytosol on Intracellular Calcium Dynamics in the Dendrite: a Simulation Study. Neurophysiology 41, 16–27 (2009). https://doi.org/10.1007/s11062-009-9072-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-009-9072-5