Abstract
Introduction
Brain metastases are a common cause of morbidity and mortality in patients with breast cancer. Local central nervous system (CNS) directed therapies are usually the first line treatment for breast cancer brain metastases (BCBM), but those must be followed by systemic therapies to achieve long-term benefit. Systemic therapy for hormone receptor (HR+) breast cancer has evolved in the last 10 years, but their role when brain metastases occur is uncertain.
Methods
We performed a systematic review of the literature focused on management of HR+ BCBM by searching Medline/PubMed, EBSCO, and Cochrane databases. The PRISMA guidelines were used for systematic review.
Results
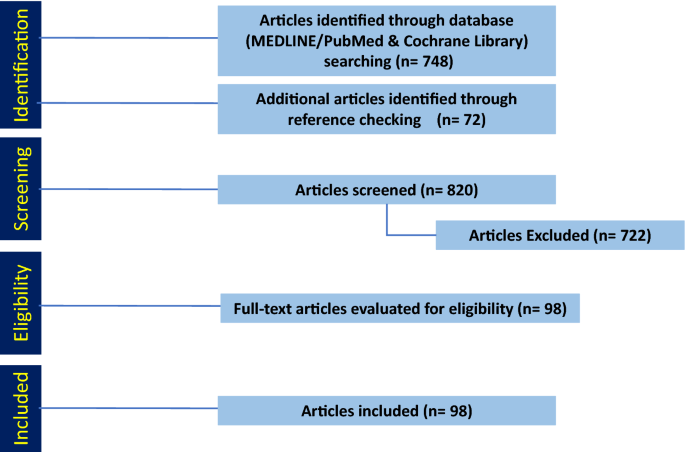
Out of 807 articles identified, 98 fulfilled the inclusion criteria in their relevance to the management of HR+ BCBM.
Conclusions
Similar to brain metastases from other neoplasms, local CNS directed therapies are the first line treatment for HR+ BCBM. Although the quality of evidence is low, after local therapies, our review supports the combination of targeted and endocrine therapies for both CNS and systemic management. Upon exhaustion of targeted/endocrine therapies, case series and retrospective reports suggest that certain chemotherapy agents are active against HR+ BCBM. Early phase clinical trials for HR+ BCBM are ongoing, but there is a need for prospective randomized trials to guide management and improve patients’ outcome.
Similar content being viewed by others
Introduction
Breast cancer is the most commonly diagnosed cancer in women worldwide, with brain metastases being a major cause of morbidity and mortality [1]. It is estimated that 10–24% of metastatic breast cancers (MBC) seed the brain (30% per autopsy series) [2,3,4], and, in the United States, it is the second most frequent malignancy to cause brain metastases [5]. Approximately 7% of patients with MBC will have brain metastases at diagnosis (synchronous) while 17% will appear later on the course of the disease (metachronous) [6, 7]. Young age, lymph node positivity, and tumor characteristics (stage, grade, size, and Ki-67 index) correlate with higher incidence of breast cancer brain metastases (BCBM) [7,8,9]. In a recent meta-analysis, BCBM were found in 15% of patients with hormone receptor positive (HR+) and about 50% of HER2+ breast cancers [10].
Several prospective trials provide evidence to support management guidelines of HER2+ BCBM [11], but for patients with HR+/HER2−, the subtype with the highest absolute incidence of brain metastases, the evidence is scant and retrospective [12]. We performed a systematic review of the published data on approved and emerging systemic treatment options that could support their use for patients with HR+ BCBM.
Methods
Literature search
We conducted a systematic literature review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13]. We queried MEDLINE/PubMed and Cochrane Library for articles published between January 1964 and June 2022 using key terms to access clinical trials and original articles on current treatment options for HR+ BCBM. The search included combinations of the following keywords “HR+ breast cancer”, “ER+ breast cancer” “brain metastases”, “surgical resection”, “radiation therapy”, “systemic therapy”, “immunotherapy”, “chemotherapy”, and “targeted therapy”. The MEDLINE/PubMed, Cochrane Library, and EBSCO Essentials databases were searched on June 25, 2022. Abstracts and presentations from national meetings from 2019 to 2022 were also searched.
Study inclusion and analysis
One author (SJ) screened all article abstracts and selected potential papers for inclusion. Another author (PD) determined if the selected papers met the inclusion/exclusion criteria. Studies were included if a primary or secondary analysis examined treatment safety or efficacy in HR+ BCBM. We excluded studies if they were not in English, were not peer reviewed, or were a letter or commentary article. Additionally, studies focused on leptomeningeal metastases were excluded. We included case reports, meta-analyses, reviews, and relevant retrospective and prospective studies that enrolled any BCBM participant with or without a pre-planned analysis of BCBM outcomes.
Findings
The search in MEDLINE/PubMed and Cochrane Library yielded 748 articles that we screened for eligibility by title and abstract (Fig. 1). Additionally, we included 59 articles that we identified in the references. Of the 820 articles, we excluded 722 that did not meet the inclusion criteria, leaving 98 included in this systematic review.
Discussion
Local therapy for HR+ BCBM
The recommendations for local therapy (surgery and radiation) for HR+ BCBM are similar to those for brain metastases from other types of cancer and previously reviewed [14]. The use of local brain directed therapy depends on the patient’s functional status, the extent of systemic extra-neural disease, the number of metastases, the neurologic symptoms, and other comorbidities. Although there are no prospective randomized studies comparing surgery and stereotactic radiosurgery (SRS) for a single brain metastasis, surgical resection is considered when complete resection with low morbidity is feasible and when there is diagnostic uncertainty, bulky disease, high symptom burden, or when a very favorable extracranial disease profile exists. Resection followed by whole brain radiotherapy (WBRT) improved survival when compared to no adjuvant post-operative radiotherapy [15, 16]. A concern associated with WBRT is the long-term effect on neurocognitive function; thus, strategies to reduce the incidence include WBRT with hippocampal avoidance (HA) [17, 18] and memantine treatment [19].
Meanwhile, SRS is often the preferred approach to treat limited volume brain metastases. Metastatic volumes greater than 10 cm3 and progressive extra-cranial disease at the time of SRS were associated with worse survival for patients with BCBM [20]. Although the indication for SRS had previously been the presence of four brain metastases or less, recent guidelines from national societies suggest that some patients with more than four brain metastases may benefit from SRS [14, 21,22,23]. Moreover, SRS has the potential to reduce the risk of long-term radiation-induced neurocognitive impairment, while improving the quality of life [24]. In most instances, a case-by-case assessment by a multidisciplinary group with consideration of risk factors is the preferred approach.
Systemic therapy
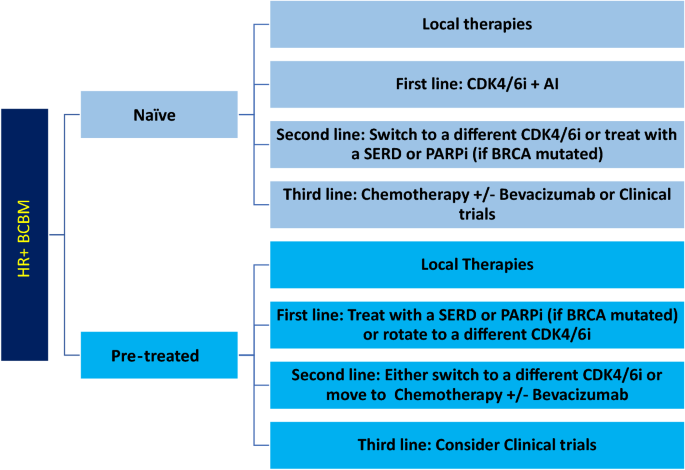
After local therapies, patients with BCBM may benefit from systemic treatment due to the high frequency of additional recurrences both in the CNS and extra-neural. For HR+ BCBM, targeted therapy is preferred for first- and second-line systemic treatments, while cytotoxic chemotherapy is reserved for later lines of treatment or cases with refractory disease (Fig. 2).
Suggested Line of Treatment for HR + BCBM. Local therapies (i.e., surgical resection and radiation) should be attempted first in naïve or pre-treated patients. Then, first, second-, and third-line systemic approach should be followed. HR + hormone receptor positive, BCBM breast cancer brain metastases, CDK4/6i cyclin dependent kinase 4/6 inhibitors, AI aromatase inhibitors, SERD selective estrogen receptor degraders, PARPi poly adenosine diphosphate-ribose polymerase inhibitors
Targeted therapy: CDK 4/6 inhibitors: palbociclib, ribociclib, and abemaciclib
Although the three FDA-approved CDK4/6i cross the blood–brain barrier (BBB), their clinical CNS efficacy is unproven. Palbociclib and abemaciclib are substrates of efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), while ribociclib is a substrate for P-gp [25]. Despite the limitations of CNS drug exposure from a pharmacologic standpoint, there are reports of clinical activity against HR+ BCBM.
Palbociclib was the first CDK4/6i approved for the treatment of HR+/HER2− MBC with or without visceral metastasis based on two randomized clinical trials [26]. Both studies allowed patients with brain metastases, but only two and five patients were accrued, respectively (no data on CNS outcome is available). The information on treatment arm for patients who developed new brain metastases while on study was not disclosed.
Ribociclib was FDA-approved based on the results from the MONALEESA-2 [27, 28] and MONALEESA-3 [29] studies. The MONALEESA-2 study excluded patients with brain metastases [28]. In the MONALEESA-3 trial, eight of the 726 patients randomized (2:1) to receive ribociclib plus fulvestrant or placebo plus fulvestrant [30] had stable brain metastases, but no specific CNS outcome data is available.
Abemaciclib was FDA-approved following the results of a phase II single arm and two randomized clinical trials [31,32,33]. However, all three studies excluded patients with brain metastases. On the other hand, a single-arm phase II study evaluated the intracranial overall response rate (ORR) in HR+ BCBM brain or leptomeningeal metastases treated with abemaciclib [34]. The patients, grouped by tumor subtype, were treated with either abemaciclib or the standard of care therapy. Despite achieving excellent CSF drug concentration with an intracranial ORR of 5.2% and an intracranial clinical benefit rate (complete responses + partial responses + stable disease) of 24% in HR+/HER2− patients, the study did not meet its primary endpoint of an intracranial ORR ≥ of 15%.
All three CDK4/6i have published case reports [35, 36] suggesting clinical activity, but there are no large controlled clinical trials demonstrating improved outcomes with these drugs for patients with HR+BCBM. Furthermore, there is scant information about appearance of new CNS metastases to draw conclusions about their ability to prevent development of BCBM. Based on their potential clinical activity and acceptable toxicity profile, an expert opinion suggested the use of CDK4/6i for patients with HR+ BCBM [37].
Data on re-treatment with CDK4/6i will be available as per the MAINTAIN clinical trial (clinicaltrials.gov NCT02632045), although this study excludes patients with active CNS metastases. Clinical trials with new CDK4/6i [dalpiciclib (NCT05586841)] and CDK2i [fadraciclib (NCT02552953)] are ongoing, but these studies exclude patients with active CNS metastases.
Endocrine therapy: tamoxifen, anastrozole, letrozole, and exemestane
Aromatase inhibitors (AI) are potentially active for the treatment of BCBM as they lower both serum and CSF concentrations of estradiol [38]. However, the only publications suggesting AI (or tamoxifen) have activity for HR+ BCBM are case series and reports [39,40,41,42,43]. Their potential efficacy is in the setting of BCBM naïve to endocrine therapies, but limited in tumors harboring ESR1 mutations or other endocrine resistance mechanisms [44]. A retrospective study of 198 patients with HR+ BCBM found that the median OS was significantly longer in patients who received endocrine therapy after a diagnosis of BCBM compared with patients who did not receive it (15 versus 4 months) [45]. Thus, for patients with newly diagnosed HR+ BCBM, it is reasonable to continue or start endocrine therapies in the setting of brain metastases, but combination therapy with a targeted agent is generally preferred.
Endocrine therapy: fulvestrant
Fulvestrant is the only FDA-approved selective estrogen receptor degrader (SERD) for breast cancer although several novel oral SERDs are in late-stage of development. Fulvestrant did not readily cross the intact BBB in animal studies [46] but two case series have suggested activity in patients with BCBM [47, 48]. The largest monotherapy fulvestrant study included patients with stable brain metastases, but outcomes for this specific group were not reported [49]. A phase II study [50] compared fulvestrant alone or in combination with capivasertib, an AKT inhibitor, in postmenopausal women with aromatase inhibitor-resistant HR+/HER2− MBC, showing a significantly longer PFS of the combination over monotherapy (10.3 versus 4.8 months, n = 71). Although patients with BCBM were included, their outcomes were not reported. There are several ongoing trials using fulvestrant alone or in combination with novel agents, which allow inclusion of patients with BCBM (Table 1).
Targeted therapy: PI3K/mTOR inhibitors
Everolimus is an mTOR inhibitor approved for late-stage HR+ MBC based on a randomized phase III trial (n = 724) [51] that suggested that the combination with exemestane offers a PFS benefit versus exemestane alone. While this study excluded BCBM, another phase II trial for BCBM, tested the CNS response rate to everolimus, trastuzumab, and vinorelbine [52] in HER2+ BCBM. The CNS response rate was 4%, the median intracranial time to progression was 3.9 months, and the median OS was 12.2 months, but the study did not meet its primary endpoint. A retrospective study of everolimus in patients with MBC and prior treatment observed a PFS of 6.8 months [53]. Nine patients with BCBM achieved a PFS of 6 months.
Alternatively, alpelisib may be an option in selected patients with PIK3CA mutations and brain metastases. Case reports (n = 4) [54] and a real world dataset with four additional cases (PFS of 43 days) [55] suggest that alpelisib may have CNS activity. Ongoing studies are examining either alpelisib or next-generation PI3K inhibitors in MBC and BCBM (NCT05230810).
Targeted therapy: PARP inhibitors
Olaparib, a PARP inhibitor with CNS penetration [56], has FDA approval in patients with MBC and a germline mutation in BRCA1 or BRCA2 genes. In an open-label phase III trial [57], monotherapy olaparib was compared with standard therapy in patients with a germline BRCA mutation and HER2− MBC. The median PFS was significantly longer in the olaparib (7.0 months) than in the standard therapy group (4.2 months), but there were no significant differences in OS [58]. This study did not report on brain metastases. Another phase II study demonstrated that olaparib is an effective and tolerable treatment in patients with MBC (brain metastases allowed) and germline PALB2 or somatic BRCA1 and BRCA2 mutations [59]; there was no report of BCBM efficacy.
Targeted therapy: bevacizumab
Bevacizumab is a vascular endothelial growth factor (VEGF) inhibitor that improved PFS in patients with MBC treated in either the first-line or the second-line setting when combined with chemotherapy [60,61,62,63,64]. However, bevacizumab ultimately had no effect on OS and the FDA indication in breast cancer was rescinded in 2011. However, phase II clinical trials [65, 66] have shown that bevacizumab may be a reasonable option as an adjuvant to cytotoxic chemotherapy in BCBM.
Chemotherapy
Existing practice guidelines for treatment of MBC recommend sequential endocrine/targeted therapy until available agents have been exhausted before deploying systemic cytotoxic chemotherapy [67]. It is unclear if this recommendation applies to BCBM. Although cytotoxic agents may be faster acting against BCBM than certain targeted/endocrine therapies, it may be at the cost of greater toxicity. Several studies that report activity for cytotoxic agents against BCBM fail to describe cohort characteristics including receptor status, undermining the establishment of their efficacy among the distinct breast cancer subtypes [68,69,70,71,72].
Capecitabine is often the first chemotherapy attempted for HR+ BCBM [10, 73], because it is thought to penetrate the BBB [74]. A retrospective study [75] and a phase I trial [69] reported responses in the brain. Likewise, methotrexate penetrates the BBB and exhibited PR (28%) responses in a retrospective study [71]. A non-randomized prospective study reported that treatment with the CMF (cyclophosphamide, methotrexate, and fluorouracil) or FAC (5-fluorouracil, doxorubicin, and cyclophosphamide) regimens led to a 59% CNS response [76]. Furthermore, a prospective study (n = 56) revealed that cisplatin and etoposide resulted in CNS response, including seven CR, 14 PR, and 12 SD [77]. Other drugs that cross the BBB and have reported clinical data include temozolomide [78], doxil [79], eribulin [80] and irinotecan [81].
Combination local and systemic therapy
The combination of chemotherapy and radiation may have synergistic effect against brain metastases. A prospective study compared the efficacy and impact on the quality of life of WBRT and chemotherapy in patients with BCBM [81]. This study randomized 58 patients stratified according to breast cancer subtypes to receive WBRT alone or WBRT plus carboplatin. The ORR was 34.4% for WBRT alone and 79.3% when combined with cisplatin. The OS (15.9 versus 11.3 months) and the PFS (10.2 versus 6.8 months) were significantly longer in the WBRT plus chemotherapy group when compared to the WBRT cohort. Karnofsky Performance Status scores significantly improved after WBRT plus chemotherapy compared to WBRT alone, while the combination had similar adverse reactions.
A phase I trial showed that bevacizumab combined with WBRT was safe and generated response in patients with brain metastases from solid tumors (n = 19), including breast cancer (n = 13) [82]. There was an 87.5% response rate at the highest dosing level (WBRT 30 Gy in 10 fractions and bevacizumab 15 mg/kg on days 1, 15, and 29).
Specifically, for patients with HR+ BCBM, a retrospective study of concurrent radiotherapy with CDK4/6i, palbociclib (n = 34) or abemaciclib (n = 2), resulted in brain metastases local control at 12 weeks of 91.7% [83]. This outcome is provocative but there is need for prospective controlled studies to support any recommendation on the combination of radiation and CDK4/6i for patients with HR+ BCBM.
Emerging therapies
Immunotherapy/antibody–drug conjugates
Immunotherapy is not approved for metastatic HR+ breast cancer (aside from rare patients with high tumor mutational burden or mismatch repair deficient cancers). A phase II (NCT02886585) study is evaluating the safety and efficacy of pembrolizumab, a checkpoint inhibitor (PD-1), in CNS metastases (brain and leptomeningeal) from multiple tumors (including breast cancer). Preliminary results from this study suggest efficacy of pembrolizumab in the treatment of leptomeningeal disease from solid tumor malignancies (n = 20, including 7 HR+/HER2- and 3 HR + /HER2+) [84], but results pertaining to brain metastases have yet to be published.
Recent phase I and II studies have shown positive results with trastuzumab deruxtecan, an antibody–drug conjugate linked to a topoisomerase I inhibitor in patients with HER2low MBC [85, 86]. A phase III trial [87] evaluated the efficacy and safety of trastuzumab deruxtecan (n = 373, HR+ = 331) in HER2low MBC patients compared to physician’s choice of chemotherapy (eribulin, capecitabine, paclitaxel, or gemcitabine) (n = 184, HR+ = 163). Trastuzumab deruxtecan significantly prolonged median PFS (10.1 versus 5.4 months) and OS (23.9 versus 17.5 months) when compared to the control arm. In the trastuzumab deruxtecan and the chemotherapy cohorts, 5.4% and 4.3% of patients had brain metastases. The brain metastases ORR was 67.4% [88] suggesting that trastuzumab deruxtecan has activity in patients with HR+, HER2low CNS metastases.
New compounds
Sacituzmab govitecan and Elacestrant received indications in HR + breast cancer in 2023 and will be studied for activity in HR + BCBM (no CNS efficacy data available to date). Multiple drugs with potential efficacy in HR+ BCBM are being studied in preclinical and clinical studies. A highlight is ANG1005, which consist of three paclitaxel molecules covalently linked to Angiopep-2 and crosses the BBB via the LRP1 (low-density lipoprotein receptor-related protein 1) transport system [89]. An open-label phase II study in BCBM (n = 72, 39 HR+) revealed an 8% intracranial ORR, better for patients with HER2+ (14%) than those with HER2− (3%).
Another phase I study [90] evaluated the optimal dose for an AKT inhibitor (MK-2206) administered in combination with anastrozole, fulvestrant, or both in postmenopausal women with HR+/HER2− MBC (n = 30). Nineteen patients had visceral involvement (including brain metastases). Preliminary results showed PR in 7.7% of the patients and a CBR of 36.7% and ORR rate of 15.4%. The most common adverse events were rash (33.3%), hyperglycemia (20%), hypophosphatemia (16.7%), and fatigue (10%).
Recommendations
There is no level 1 evidence based on prospective randomized clinical trials to provide guidance on systemic therapies for HR+ BCBM. The current potentially effective first-line systemic therapies for HR+ BCBM, (Fig. 2) include CDK4/6i (palbociclib, ribociclib, or abemaciclib) in combination with aromatase inhibitors, or SERDs. Potential options for second-line systemic treatments include trastuzumab deruxtecan if HER2low, CDK4/6i rotation, a mTORC1 inhibitor, a PARP inhibitor if BRCA mutated, or other molecularly targeted inhibitors such as alpelisib (usually given with an endocrine agent). Pre-treated patients may have endocrine resistance (i.e., ESR1 mutation), thus, a personalized approach based on molecular testing may be of benefit. Upon exhaustion of targeted/endocrine therapies, chemotherapy agents such as capecitabine, trastuzumab deruxtecan, eribulin or others (with or without bevacizumab) could be an option (Table 2).
Expert opinions/recommendation in the area of HR+BCBM are limited since many published studies fail to disclose the receptor status or to make direct correlations between receptor status, brain metastases, and treatment response. Furthermore, at least 20% of BCBM have receptors that differ from the primary cancer [91,92,93,94,95,96,97].
Conclusion
Despite the advances in systemic therapies for HR+ breast cancer, the treatment of brain metastases remains a major therapeutic challenge that requires a multidisciplinary approach. The contemporary recommendations for the treatment of HR+ BCBM involve local therapies; maximal local control with surgery, SRS and WBRT with the option of repeated local therapy for recurrence whenever feasible [14].
Clinical trials are increasingly available for patients with BCBM (Table 1), but the field needs randomized clinical trials of new drug candidates that include patients with BCBM and report separately on their outcomes. Research into distinct biomarkers BCBM that could aid in early detection and improve personalized targeted therapy is needed. Screening for brain metastases in patients with MBC is not generally recommended; however, approximately 20% [98] of patients with BCBM are asymptomatic. Asymptomatic patients have less CNS metastatic burden and better outcomes than patients who are symptomatic [99]. Noninvasive techniques such as liquid biopsy presents an emerging aspect of breast cancer care that may help improve future CNS surveillance.
Survival from HR+ breast cancer is improving as drugs that are more effective become available, but as patients with MBC live longer, the likelihood of CNS relapse increases. The recommendations for local therapies are robust, but systemic therapy recommendation are limited by the quality of evidence. There is urgency to study new and potentially more effective therapies in well-designed, clinical trials to improve outcomes of the growing population with breast cancer and brain metastases.
Data Availability
Not applicable
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, Mouret-Reynier MA, Goncalves A, Dalenc F, Delaloge S, Campone M, Augereau P, Ferrero JM, Levy C, Fumet JD, Lecouillard I, Cottu P, Petit T, Uwer L, Jouannaud C, Leheurteur M, Dieras V, Robain M, Chevrot M, Pasquier D, Bachelot T (2019) Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer 121:991–1000. https://doi.org/10.1038/s41416-019-0619-y
Tsukada Y, Fouad A, Pickren JW, Lane WW (1983) Central nervous system metastasis from breast carcinoma. Autopsy study Cancer 52:2349–2354. https://doi.org/10.1002/1097-0142(19831215)52:12%3c2349::aid-cncr2820521231%3e3.0.co;2-b
Khan M, Arooj S, Li R, Tian Y, Zhang J, Lin J, Liang Y, Xu A, Zheng R, Liu M, Yuan Y (2020) Tumor primary site and histology subtypes role in radiotherapeutic management of brain metastases. Front Oncol 10:781. https://doi.org/10.3389/fonc.2020.00781
Van Mechelen M, Van Herck A, Punie K, Nevelsteen I, Smeets A, Neven P, Weltens C, Han S, Vanderstichele A, Floris G, Lobelle JP, Wildiers H (2020) Behavior of metastatic breast cancer according to subtype. Breast Cancer Res Treat 181:115–125. https://doi.org/10.1007/s10549-020-05597-3
Koniali L, Hadjisavvas A, Constantinidou A, Christodoulou K, Christou Y, Demetriou C, Panayides AS, Pitris C, Pattichis CS, Zamba-Papanicolaou E, Kyriacou K (2020) Risk factors for breast cancer brain metastases: a systematic review. Oncotarget 11:650–669. https://doi.org/10.18632/oncotarget.27453
Graesslin O, Abdulkarim BS, Coutant C, Huguet F, Gabos Z, Hsu L, Marpeau O, Uzan S, Pusztai L, Strom EA, Hortobagyi GN, Rouzier R, Ibrahim NK (2010) Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 28:2032–2037. https://doi.org/10.1200/JCO.2009.24.6314
Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, Valabrega G, Aglietta M, Montemurro F (2014) Metastatic breast cancer subtypes and central nervous system metastases. Breast 23:623–628. https://doi.org/10.1016/j.breast.2014.06.009
Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, Dhaliwal AJ, Sahgal A, Das S, Chan KK, Jerzak KJ (2021) The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol 23:894–904. https://doi.org/10.1093/neuonc/noaa285
Ramakrishna N, Anders CK, Lin NU, Morikawa A, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Franzoi MAB, Kirshner JJ, Krop IE, Patt DA, Perlmutter J, Giordano SH (2022) Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol 40:2636–2655. https://doi.org/10.1200/JCO.22.00520
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA, Alexander BM, Lin NU, Aizer AA (2017) Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 3:1069–1077. https://doi.org/10.1001/jamaoncol.2017.0001
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D (2022) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40:492–516. https://doi.org/10.1200/JCO.21.02314
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/NEJM199002223220802
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583–590. https://doi.org/10.1002/ana.410330605
Han YM, Cai G, Chai WM, Xu C, Cao L, Ou D, Chen JY, Kirova YM (2017) Radiological distribution of brain metastases and its implication for the hippocampus avoidance in whole brain radiotherapy approach. Br J Radiol 90:20170099. https://doi.org/10.1259/bjr.20170099
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816. https://doi.org/10.1200/JCO.2014.57.2909
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, Radiation Therapy Oncology G (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 15:1429–1437. https://doi.org/10.1093/neuonc/not114
Wilson TG, Robinson T, MacFarlane C, Spencer T, Herbert C, Wade L, Reed H, Braybrooke JP (2020) Treating brain metastases from breast cancer: outcomes after stereotactic radiosurgery. Clin Oncol (R Coll Radiol) 32:390–396. https://doi.org/10.1016/j.clon.2020.02.007
Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Giordano SH, Gonzalez-Angulo AM, Kirshner JJ, Krop I, Levinson J, Modi S, Patt DA, Perez EA, Perlmutter J, Winer EP, Lin NU (2014) Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:2100–2108. https://doi.org/10.1200/JCO.2013.54.0955
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225. https://doi.org/10.1016/j.prro.2011.12.004
Grandhi R, Kondziolka D, Panczykowski D, Monaco EA 3rd, Kano H, Niranjan A, Flickinger JC, Lunsford LD (2012) Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg 117:237–245. https://doi.org/10.3171/2012.4.JNS11870
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Groenland SL, Martinez-Chavez A, van Dongen MGJ, Beijnen JH, Schinkel AH, Huitema ADR, Steeghs N (2020) Clinical Pharmacokinetics and Pharmacodynamics of the Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib, and Abemaciclib. Clin Pharmacokinet 59:1501–1520. https://doi.org/10.1007/s40262-020-00930-x
Turner NC, Finn RS, Martin M, Im SA, DeMichele A, Ettl J, Dieras V, Moulder S, Lipatov O, Colleoni M, Cristofanilli M, Lu DR, Mori A, Giorgetti C, Iyer S, Bartlett CH, Gelmon KA (2018) Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol 29:669–680. https://doi.org/10.1093/annonc/mdx797
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738–1748. https://doi.org/10.1056/NEJMoa1609709
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell KL, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Mondal S, Su F, Miller M, Elmeliegy M, Germa C, O’Shaughnessy J (2018) Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 29:1541–1547. https://doi.org/10.1093/annonc/mdy155
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Pivot X, Vidam G, Wang Y, Rodriguez Lorenc K, Miller M, Taran T, Jerusalem G (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465–2472. https://doi.org/10.1200/JCO.2018.78.9909
Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, Petrakova K, Valeria Bianchi G, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Ji Y, Wang C, Deore U, Chakravartty A, Zarate JP, Taran T, Fasching PA (2021) Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 32:1015–1024. https://doi.org/10.1016/j.annonc.2021.05.353
Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, Wildiers H, Hudis CA, O’Shaughnessy J, Zamora E, Yardley DA, Frenzel M, Koustenis A, Baselga J (2017) MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res 23:5218–5224. https://doi.org/10.1158/1078-0432.CCR-17-0754
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Conte P, Lu Y, Barriga S, Hurt K, Frenzel M, Johnston S, Llombart-Cussac A (2020) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 6:116–124. https://doi.org/10.1001/jamaoncol.2019.4782
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, Yardley D, Chan A, Conte P, Dieras V, Lin NU, Bear M, Chapman SC, Yang Z, Chen Y, Anders CK (2020) A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res 26:5310–5319. https://doi.org/10.1158/1078-0432.CCR-20-1764
Troussier I, Canova C, Klausner G (2020) Complete response of leptomeningeal carcinomatosis secondary to breast cancer. Breast 54:328–330. https://doi.org/10.1016/j.breast.2020.11.019
Radke I, von Wahlde MK, Schulke C, Tio J (2020) Ribociclib in breast cancer brain metastases: a case report. Breast Care (Basel) 15:543–547. https://doi.org/10.1159/000504405
Schlam I, Tolaney SM (2021) Is there a role for CDK 4/6 inhibitors in breast cancer brain metastases? Oncotarget 12:873–875. https://doi.org/10.18632/oncotarget.27904
Azcoitia I, Mendez P, Garcia-Segura LM (2021) Aromatase in the human brain. Androg Clin Res Ther 2:189–202. https://doi.org/10.1089/andro.2021.0007
Madhup R, Kirti S, Bhatt ML, Srivastava PK, Srivastava M, Kumar S (2006) Letrozole for brain and scalp metastases from breast cancer—a case report. Breast 15:440–442. https://doi.org/10.1016/j.breast.2005.07.006
Goyal S, Puri T, Julka PK, Rath GK (2008) Excellent response to letrozole in brain metastases from breast cancer. Acta Neurochir (Wien) 150:613–614. https://doi.org/10.1007/s00701-008-1576-z. (discussion 614-615)
Ito K, Ito T, Okada T, Watanabe T, Gomi K, Kanai T, Mochizuki Y, Amano J (2009) A case of brain metastases from breast cancer that responded to anastrozole monotherapy. Breast J 15:435–437. https://doi.org/10.1111/j.1524-4741.2009.00756.x
Almajed MM, Esfahani K, Pelmus M, Panasci L (2016) Complete response and long-term survival of leptomeningeal carcinomatosis from breast cancer with maintenance endocrine therapy. BMJ Case Rep. https://doi.org/10.1136/bcr-2016-215525
Saha P, Amico AL, Olopade OI (2016) Long-term disease-free survival in a young patient with hormone receptor-positive breast cancer and oligometastatic disease in the brain. Clin Breast Cancer 16:e61-63. https://doi.org/10.1016/j.clbc.2016.02.013
Turner NC, Swift C, Kilburn L, Fribbens C, Beaney M, Garcia-Murillas I, Budzar AU, Robertson JFR, Gradishar W, Piccart M, Schiavon G, Bliss JM, Dowsett M, Johnston SRD, Chia SK (2020) ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res 26:5172–5177. https://doi.org/10.1158/1078-0432.CCR-20-0224
Bergen ES, Berghoff AS, Medjedovic M, Rudas M, Fitzal F, Bago-Horvath Z, Dieckmann K, Mader RM, Exner R, Gnant M, Zielinski CC, Steger GG, Preusser M, Bartsch R (2019) Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res 25:2737–2744. https://doi.org/10.1158/1078-0432.CCR-18-1968
Howell A, Osborne CK, Morris C, Wakeling AE (2000) ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer 89:817–825. https://doi.org/10.1002/1097-0142(20000815)89:4%3c817::aid-cncr14%3e3.0.co;2-6
Wang Q, Sun B, Liu C, Shi S, Ding L, Liu J, Wu S (2019) Brain metastases from breast cancer may respond to endocrine therapy: report of two cases. Onco Targets Ther 12:1389–1393. https://doi.org/10.2147/OTT.S188143
Rusz O, Koszo R, Dobi A, Csenki M, Valicsek E, Nikolenyi A, Uhercsak G, Cserhati A, Kahan Z (2018) Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: a case series. Onco Targets Ther 11:5459–5463. https://doi.org/10.2147/OTT.S170736
Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, Shparyk Y, Cardona-Huerta S, Cheung KL, Philco-Salas MJ, Ruiz-Borrego M, Shao Z, Noguchi S, Rowbottom J, Stuart M, Grinsted LM, Fazal M, Ellis MJ (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388:2997–3005. https://doi.org/10.1016/S0140-6736(16)32389-3
Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, Madden TA, Bale C, Bezecny P, Joffe J, Moon S, Twelves C, Venkitaraman R, Waters S, Foxley A, Howell SJ (2020) Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 21:345–357. https://doi.org/10.1016/S1470-2045(19)30817-4
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529. https://doi.org/10.1056/NEJMoa1109653
Van Swearingen AED, Siegel MB, Deal AM, Sambade MJ, Hoyle A, Hayes DN, Jo H, Little P, Dees EC, Muss H, Jolly T, Zagar TM, Patel N, Miller CR, Parker JS, Smith JK, Fisher J, Shah N, Nabell L, Nanda R, Dillon P, Abramson V, Carey LA, Anders CK (2018) LCCC 1025: a phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res Treat 171:637–648. https://doi.org/10.1007/s10549-018-4852-5
Shen XB, Li GL, Zheng YB, Chen ZH, Cao WM, Wang XJ, Shao XY (2021) Combined everolimus and endocrine therapy in advanced HR-positive, HER2-negative Chinese breast cancer patients: a retrospective study. Ann Transl Med 9:1334. https://doi.org/10.21037/atm-21-3840
Batalini F, Moulder SL, Winer EP, Rugo HS, Lin NU, Wulf GM (2020) Response of brain metastases from PIK3CA-mutant breast cancer to alpelisib. JCO Precis Oncol. https://doi.org/10.1200/PO.19.00403
Miller J, Armgardt E, Svoboda A (2022) The efficacy and safety of alpelisib in breast cancer: a real-world analysis. J Oncol Pharmacy Pract. https://doi.org/10.1177/10781552221096413
Song YK, Kim MJ, Kim MS, Lee JH, Chung SJ, Song JS, Chae YJ, Lee KR (2022) Role of the efflux transporters Abcb1 and Abcg2 in the brain distribution of olaparib in mice. Eur J Pharm Sci 173:106177. https://doi.org/10.1016/j.ejps.2022.106177
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523–533. https://doi.org/10.1056/NEJMoa1706450
Robson M, Ruddy KJ, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Li W, Tung N, Armstrong A, Delaloge S, Bannister W, Goessl C, Degboe A, Hettle R, Conte P (2019) Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer 120:20–30. https://doi.org/10.1016/j.ejca.2019.06.023
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, Shah PD, Ballinger TJ, Yang ES, Vinayak S, Melisko M, Brufsky A, DeMeo M, Jenkins C, Domchek S, D’Andrea A, Lin NU, Hughes ME, Carey LA, Wagle N, Wulf GM, Krop IE, Wolff AC, Winer EP, Garber JE (2020) TBCRC 048: Phase II study of Olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38:4274–4282. https://doi.org/10.1200/JCO.20.02151
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676. https://doi.org/10.1056/NEJMoa072113
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:3239–3247. https://doi.org/10.1200/JCO.2008.21.6457
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29:1252–1260. https://doi.org/10.1200/JCO.2010.28.0982
Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, Masuda N, Ro J, Denduluri N, Hubeaux S, Quah C, Bais C, O’Shaughnessy J (2017) Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer 70:146–155. https://doi.org/10.1016/j.ejca.2016.09.024
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29:4286–4293. https://doi.org/10.1200/JCO.2010.34.1255
Leone JP, Emblem KE, Weitz M, Gelman RS, Schneider BP, Freedman RA, Younger J, Pinho MC, Sorensen AG, Gerstner ER, Harris G, Krop IE, Morganstern D, Sohl J, Hu J, Kasparian E, Winer EP, Lin NU (2020) Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res 22:131. https://doi.org/10.1186/s13058-020-01372-w
Lu YS, Chen TW, Lin CH, Yeh DC, Tseng LM, Wu PF, Rau KM, Chen BB, Chao TC, Huang SM, Huang CS, Shih TT, Cheng AL, Taiwan Breast Cancer C (2015) Bevacizumab preconditioning followed by Etoposide and Cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin Cancer Res 21:1851–1858. https://doi.org/10.1158/1078-0432.CCR-14-2075
Lin NU, Gaspar LE, Soffietti R (2017) Breast cancer in the central nervous system: multidisciplinary considerations and management. Am Soc Clin Oncol Educ Book 37:45–56. https://doi.org/10.14694/EDBK_175338. (10.1200/EDBK_175338)
Rosner D, Nemoto T, Lane WW (1986) Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer 58:832–839. https://doi.org/10.1002/1097-0142(19860815)58:4%3c832::aid-cncr2820580404%3e3.0.co;2-w
Rivera E, Meyers C, Groves M, Valero V, Francis D, Arun B, Broglio K, Yin G, Hortobagyi GN, Buchholz T (2006) Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 107:1348–1354. https://doi.org/10.1002/cncr.22127
Wang ML, Yung WK, Royce ME, Schomer DF, Theriault RL (2001) Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. Am J Clin Oncol 24:421–424. https://doi.org/10.1097/00000421-200108000-00026
Lassman AB, Abrey LE, Shah GD, Panageas KS, Begemann M, Malkin MG, Raizer JJ (2006) Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol 78:255–260. https://doi.org/10.1007/s11060-005-9044-6
Vinolas N, Graus F, Mellado B, Caralt L, Estape J (1997) Phase II trial of cisplatinum and etoposide in brain metastases of solid tumors. J Neurooncol 35:145–148. https://doi.org/10.1023/a:1005835430489
Bailleux C, Eberst L, Bachelot T (2021) Treatment strategies for breast cancer brain metastases. Br J Cancer 124:142–155. https://doi.org/10.1038/s41416-020-01175-y
Mata JF, Garcia-Manteiga JM, Lostao MP, Fernandez-Veledo S, Guillen-Gomez E, Larrayoz IM, Lloberas J, Casado FJ, Pastor-Anglada M (2001) Role of the human concentrative nucleoside transporter (hCNT1) in the cytotoxic action of 5[Prime]-deoxy-5-fluorouridine, an active intermediate metabolite of capecitabine, a novel oral anticancer drug. Mol Pharmacol 59:1542–1548. https://doi.org/10.1124/mol.59.6.1542
Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE (2007) Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol 85:223–227. https://doi.org/10.1007/s11060-007-9409-0
Boogerd W, Dalesio O, Bais EM, van der Sande JJ (1992) Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 69:972–980. https://doi.org/10.1002/1097-0142(19920215)69:4%3c972::aid-cncr2820690423%3e3.0.co;2-p
Franciosi V, Cocconi G, Michiara M, Di Costanzo F, Fosser V, Tonato M, Carlini P, Boni C, Di Sarra S (1999) Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer 85:1599–1605
Trudeau ME, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, Eisenhauer E (2006) Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann Oncol 17:952–956. https://doi.org/10.1093/annonc/mdl056
Linot B, Campone M, Augereau P, Delva R, Abadie-Lacourtoisie S, Nebout-Mesgouez N, Capitain O (2014) Use of liposomal doxorubicin-cyclophosphamide combination in breast cancer patients with brain metastases: a monocentric retrospective study. J Neurooncol 117:253–259. https://doi.org/10.1007/s11060-014-1378-5
Fabi A, Terrenato I, Vidiri A, Villani V, Tanzilli A, Airoldi M, Pedani F, Magri V, Palleschi M, Donadio M, Catania G, Nistico C, Carapella C, Ruda R, Pace A, Maschio M, Telera S, Cognetti F, Aino (2021) Eribulin in brain metastases of breast cancer: outcomes of the EBRAIM prospective observational trial. Future Oncol 17:3445–3456. https://doi.org/10.2217/fon-2021-0300
Kai Xu YH, Yao J, Zhong Xu, He X (2021) Whole-brain radiotherapy and chemotherapy in the treatment of patients with breast cancer and brain metastases. Int J Clin Exp Med 14:1250–1257
Levy C, Allouache D, Lacroix J, Dugue AE, Supiot S, Campone M, Mahe M, Kichou S, Leheurteur M, Hanzen C, Dieras V, Kirova Y, Campana F, Le Rhun E, Gras L, Bachelot T, Sunyach MP, Hrab I, Geffrelot J, Gunzer K, Constans JM, Grellard JM, Clarisse B, Paoletti X (2014) REBECA: a phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann Oncol 25:2351–2356. https://doi.org/10.1093/annonc/mdu465
Kim KN, Shah P, Clark A, Freedman GM, Dastgheyb S, Barsky AR, Dreyfuss AD, Taunk NK (2021) Safety of cyclin-dependent kinase4/6 inhibitor combined with palliative radiotherapy in patients with metastatic breast cancer. Breast 60:163–167. https://doi.org/10.1016/j.breast.2021.10.001
Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, Chukwueke U, White MD, Nayyar N, Kim A, Alvarez-Breckenridge C, Krop I, Mahar MK, Bertalan MS, Shaw B, Mora JL, Goss N, Subramanian M, Nayak L, Dietrich J, Forst DA, Nahed BV, Batchelor TT, Shih HA, Gerstner ER, Moy B, Lawrence D, Giobbie-Hurder A, Carter SL, Oh K, Cahill DP, Sullivan RJ (2020) Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med 26:1280–1284. https://doi.org/10.1038/s41591-020-0918-0
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T (2016) DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097–5108. https://doi.org/10.1158/1078-0432.CCR-15-2822
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T (2016) Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 107:1039–1046. https://doi.org/10.1111/cas.12966
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga JY, Im SA, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim SB, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA, Investigators DE-BT (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Hurvitz S ea (2021) Trastuzumab deruxtecan (T-DXd; DS-8201a) vs trastuzumab emtansine (T-DM1) in patients with HER2+ metastatic breast cancer., 2021 San Antonio Breast Cancer Symposium
Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, Carrillo J, Chalasani P, Kabos P, Puhalla S, Tkaczuk K, Garcia AA, Ahluwalia MS, Wefel JS, Lakhani N, Ibrahim N (2020) ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin Cancer Res 26:2789–2799. https://doi.org/10.1158/1078-0432.CCR-19-3258
Ma CX, Sanchez C, Gao F, Crowder R, Naughton M, Pluard T, Creekmore A, Guo Z, Hoog J, Lockhart AC, Doyle A, Erlichman C, Ellis MJ (2016) A phase I study of the AKT inhibitor MK-2206 in combination with hormonal therapy in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin Cancer Res 22:2650–2658. https://doi.org/10.1158/1078-0432.CCR-15-2160
Bachmann C, Grischke EM, Fehm T, Staebler A, Schittenhelm J, Wallwiener D (2013) CNS metastases of breast cancer show discordant immunohistochemical phenotype compared to primary. J Cancer Res Clin Oncol 139:551–556. https://doi.org/10.1007/s00432-012-1358-0
Bachmann C, Grischke EM, Staebler A, Schittenhelm J, Wallwiener D (2013) Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J Cancer Res Clin Oncol 139:1909–1916. https://doi.org/10.1007/s00432-013-1511-4
Kaidar-Person O, Meattini I, Jain P, Bult P, Simone N, Kindts I, Steffens R, Weltens C, Navarria P, Belkacemi Y, Lopez-Guerra J, Livi L, Baumert BG, Vieites B, Limon D, Kurman N, Ko K, Yu JB, Chiang V, Poortmans P, Zagar T (2018) Discrepancies between biomarkers of primary breast cancer and subsequent brain metastases: an international multicenter study. Breast Cancer Res Treat 167:479–483. https://doi.org/10.1007/s10549-017-4526-8
Sava A, Costea CF, Vatavu R, Grigore M, Turliuc MD, Dumitrescu GF, Eva L, Motoc AGM, Stan CI, Gavril LC, Scripcariu SI (2021) Brain metastases originating in breast cancer: clinical-pathological analysis and immunohistochemical profile. Rom J Morphol Embryol 62:435–444. https://doi.org/10.47162/RJME.62.2.09
Timmer M, Werner JM, Rohn G, Ortmann M, Blau T, Cramer C, Stavrinou P, Krischek B, Mallman P, Goldbrunner R (2017) Discordance and conversion rates of progesterone-, estrogen-, and HER2/neu-receptor status in primary breast cancer and brain metastasis mainly triggered by hormone therapy. Anticancer Res 37:4859–4865. https://doi.org/10.21873/anticanres.11894
Kotecha R, Tonse R, Rubens M, McDermott MW, Odia Y, Appel H, Mehta MP (2021) Systematic review and meta-analysis of breast cancer brain metastasis and primary tumor receptor expression discordance. Neurooncol Adv 3:vdab010. https://doi.org/10.1093/noajnl/vdab010
Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava R, Hamilton RL, Chmielecki J, Puhalla SL, Davidson NE, Oesterreich S, Brufsky AM, Young L, Lee AV (2017) Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol 3:666–671. https://doi.org/10.1001/jamaoncol.2016.5630
Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H (2014) Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 147:103–112. https://doi.org/10.1007/s10549-014-3090-8
Laakmann E, Witzel I, Neunhoffer T, Weide R, Schmidt M, Park-Simon TW, Mobus V, Mundhenke C, Polasik A, Lubbe K, Hesse T, Riecke K, Thill M, Fasching PA, Denkert C, Fehm T, Nekljudova V, Rey J, Loibl S, Muller V (2020) Characteristics and clinical outcome of breast cancer patients with asymptomatic brain metastases. Cancers (Basel). https://doi.org/10.3390/cancers12102787
Acknowledgements
The authors thank the University of Virginia Summer Medical Research Internship Program for support.
Funding
The authors declare that a support was received from the University of Virginia Summer Medical Research Internship Program. No other funds, grants or other support were received during the preparation of the manuscript. This research was supported by Office of Extramural Research, National Institutes of Health (Grant 2P30CA044579-26, 2P30CA044579-26).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by SJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jusino, S., Fadul, C.E. & Dillon, P. Systematic review of the management of brain metastases from hormone receptor positive breast cancer. J Neurooncol 162, 45–57 (2023). https://doi.org/10.1007/s11060-023-04276-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04276-9