Abstract
Background
Amplification of EGFR and its active mutant EGFRvIII are common in glioblastoma (GB). While EGFR and EGFRvIII play critical roles in pathogenesis, targeted therapy with EGFR-tyrosine kinase inhibitors or antibodies has shown limited efficacy. To improve the likelihood of effectiveness, we targeted adult patients with recurrent GB enriched for simultaneous EGFR amplification and EGFRvIII mutation, with osimertinib/bevacizumab at doses described for non-small cell lung cancer.
Methods
We retrospectively explored whether previously described EGFRvIII mutation in association with EGFR gene amplification could predict response to osimertinib/bevacizumab combination in a subset of 15 patients treated at recurrence. The resistance pattern in a subgroup of subjects is described using a commercial next-generation sequencing panel in liquid biopsy.
Results
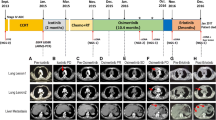
There were ten males (66.7%), and the median patient’s age was 56 years (range 38–70 years). After their initial diagnosis, 12 patients underwent partial (26.7%) or total resection (53.3%). Subsequently, all cases received IMRT and concurrent and adjuvant temozolomide (TMZ; the median number of cycles 9, range 6–12). The median follow-up after recurrence was 17.1 months (95% CI 12.3–22.6). All patients received osimertinib/bevacizumab as a second-line intervention with a median progression-free survival (PFS) of 5.1 months (95% CI 2.8–7.3) and overall survival of 9.0 months (95% CI 3.9–14.0). The PFS6 was 46.7%, and the overall response rate was 13.3%. After exposure to the osimertinib/bevacizumab combination, the main secondary alterations were MET amplification, STAT3, IGF1R, PTEN, and PDGFR.
Conclusions
While the osimertinib/bevacizumab combination was marginally effective in most GB patients with simultaneous EGFR amplification plus EGFRvIII mutation, a subgroup experienced a long-lasting meaningful benefit. The findings of this brief cohort justify the continuation of the research in a clinical trial. The pattern of resistance after exposure to osimertinib/bevacizumab includes known mechanisms in the regulation of EGFR, findings that contribute to the understanding and targeting in a stepwise rational this pathway.





Similar content being viewed by others
Data availability
The datasets presented in this article are not readily available because of the Colombian organic law of data protection that limits access to genetic information in an open format. Requests to access the datasets should be directed to the corresponding author, who will release it upon formal request to the Ministry of Health of Colombia following the requirements of Law 1581 of 2012, paragraph 201811601170851 of 2018.
References
Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. https://doi.org/10.1093/neuonc/noab106
Weller M, van den Bent M, Preusser M et al (2020) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186. https://doi.org/10.1038/s41571-020-00447-z
McNeill KA (2016) Epidemiology of brain tumors. Neurol Clin 34(4):981–998. https://doi.org/10.1016/j.ncl.2016.06.014
Chen W, Wang Y, Zhao B et al (2021) Optimal therapies for recurrent glioblastoma: a bayesian network meta-analysis. Front Oncol 29(11):641878. https://doi.org/10.3389/fonc.2021.641878
McBain C, Lawrie TA, Rogozińska E et al (2021) Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev (1). Art. No.: CD013579. https://doi.org/10.1002/14651858.CD013579.pub2
Birzu C, French P, Caccese M et al (2020) Recurrent glioblastoma: from molecular landscape to new treatment perspectives. Cancers (Basel) 13(1):47. https://doi.org/10.3390/cancers13010047
Vartanian A, Singh SK, Agnihotri S et al (2014) GBM’s multifaceted landscape: highlighting regional and microenvironmental heterogeneity. Neuro Oncol 16(9):1167–1175. https://doi.org/10.1093/neuonc/nou035
Zhao YH, Wang ZF, Pan ZY et al (2019) A meta-analysis of survival outcomes following reoperation in recurrent glioblastoma: time to consider the timing of reoperation. Front Neurol 26(10):286. https://doi.org/10.3389/fneur.2019.00286
Wang Y, Xing D, Zhao M et al (2016) The role of a single angiogenesis inhibitor in the treatment of recurrent glioblastoma multiforme: a meta-analysis and systematic review. PLoS One 11(3):e0152170. https://doi.org/10.1371/journal.pone.0152170
Kazmi F, Soon YY, Leong YH et al (2019) Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol 142(1):79–90. https://doi.org/10.1007/s11060-018-03064-0
Brat DJ, Aldape K, Colman H et al (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol 136(5):805–810. https://doi.org/10.1007/s00401-018-1913-0
Brat DJ, Aldape K, Colman H (2020) cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol 139(3):603–608. https://doi.org/10.1007/s00401-020-02127-9
Tesileanu CMS, Dirven L, Wijnenga MMJ et al (2020) Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol 22(4):515–523. https://doi.org/10.1093/neuonc/noz200
Sahm F, Schrimpf D, Jones DT et al (2016) Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol 131(6):903–910. https://doi.org/10.1007/s00401-015-1519-8
Zacher A, Kaulich K, Stepanow S et al (2017) Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol 27(2):146–159. https://doi.org/10.1111/bpa.12367
Capper D, Jones DTW, Sill M et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555(7697):469–474. https://doi.org/10.1038/nature26000
Stichel D, Schrimpf D, Casalini B et al (2019) Routine RNA sequencing of formalin-fixed paraffin-embedded specimens in neuropathology diagnostics identifies diagnostically and therapeutically relevant gene fusions. Acta Neuropathol 138(5):827–835. https://doi.org/10.1007/s00401-019-02039-3
Singh B, Coffey RJ (2014) Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annu Rev Physiol 76(1):275–300
Mayer BJ (2015) The discovery of modular binding domains: building blocks of cell signalling. Nat Rev Mol Cell Biol 16:691–698
Thorpe LM, Yuzugullu H, Zhao JJ (2015) PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 15:7–24
Verhaak RG, Hoadley KA, Purdom E et al Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110
Brennan CW, Verhaak RG, McKenna A et al TCGA Research Network (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477
Furnari FB, Cloughesy TF, Cavenee WK et al (2015) Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat Rev Cancer 15(5):302–310
Eskilsson E, Rosland GV, Talasila KM et al (2016) EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro Oncol 18(12):1644–1655
An Z, Aksoy O, Zheng T et al (2018) Epidermal growth factor receptor (EGFR) and EGFRvIII in glioblastoma (GB): signaling pathways and targeted therapies. Oncogene 37(12):1561–1575
Villano JL, Mauer AM, Vokes EE (2003) A case study documenting the anticancer activity of ZD1839 (Iressa) in the brain. Ann Oncol 14(4):656–658. https://doi.org/10.1093/annonc/mdg153
Yang J, Yan J, Liu B (2017) Targeting EGFRvIII for glioblastoma multiforme. Cancer Lett 403:224–230. https://doi.org/10.1016/j.canlet.2017.06.024
Chi AS, Cahill DP, Reardon DA et al (2020) Exploring predictors of response to dacomitinib in EGFR-amplified recurrent glioblastoma. JCO Precis Oncol 4:PO.19.00295
Lee A, Arasaratnam M, Chan DLH et al (2020) Anti-epidermal growth factor receptor therapy for glioblastoma in adults. Cochrane Database Syst Rev 5(5):CD013238. https://doi.org/10.1002/14651858.CD013238.pub2
Liu X, Chen X, Shi L et al (2019) The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J Exp Clin Cancer Res 38(1):219. https://doi.org/10.1186/s13046-019-1235-7
Chagoya G, Kwatra SG, Nanni CW et al (2020) Efficacy of osimertinib against EGFRvIII+ glioblastoma. Oncotarget 11(22):2074–2082. https://doi.org/10.18632/oncotarget.27599
Luger AL, Lorenz NI, Urban H et al (2020) Activation of epidermal growth factor receptor sensitizes glioblastoma cells to hypoxia-induced cell death. Cancers 12:2144. https://doi.org/10.3390/cancers12082144
Wen PY, Chang SM, Van den Bent MJ et al (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35(21):2439–2449
World Medical Association (2020) WMA Declaration of Helsinki: ethical principles for medical research involving human subjects. 64th WMA General Assembly, Fortaleza, Brazil. October 2013. Accessed 30 Nov 2020. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
Frampton GM, Fichtenholtz A, Otto GA et al (2013) Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 31:1023–1031
Palmisano WA, Divine KK, Saccomanno G et al (2000) Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res 60:5954–5958
Yoshimoto K, Dang J, Zhu S et al (2008) Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res 14:488–493
Idbaih A, Aimard J, Boisselier B et al (2009) Epidermal growth factor receptor extracellular domain mutations in primary glioblastoma. Neuropathol Appl Neurobiol 35:208–213
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Ichimura K, Pearson DM, Kocialkowski S et al (2009) IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro-Oncology 11:341–347
Taal W, Oosterkamp HM, Walenkamp AM et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15(9):943–953. https://doi.org/10.1016/S1470-2045(14)70314-6
Lombardi G, De Salvo GL, Brandes AA et al (2019) Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 20(1):110–119. https://doi.org/10.1016/S1470-2045(18)30675-2
Kesari S, Ram Z, EF-14 Trial Investigators (2017) Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial. CNS Oncol 6(3):185–193. https://doi.org/10.2217/cns-2016-0049
Chen JR, Xu HZ, Yao Y et al (2015) Prognostic value of epidermal growth factor receptor amplification and EGFRvIII in glioblastoma: meta-analysis. Acta Neurol Scand 132(5):310–322
Wang N, Zhang Y, Mi Y et al (2020) Osimertinib for EGFR-mutant lung cancer with central nervous system metastases: a meta-analysis and systematic review. Ann Palliat Med 9(5):3038–3047. https://doi.org/10.21037/apm-20-605
Ekman S, Cselényi Z, Varrone A et al (2021) A PET and MRI study exploring osimertinib brain exposure and efficacy in EGFRm NSCLC CNS metastases. Presented at: 2020 World Lung Conference on Lung cancer Singapore, January 28–31, 2021, Virtual. Poster P76.72
Cross DA, Ashton SE, Ghiorghiu S et al (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4(9):1046–1061
Makhlin I, Salinas RD, Zhang D et al (2019) Clinical activity of the EGFR> tyrosine kinase inhibitor osimertinib in EGFR-mutant glioblastoma. CNS Oncol 8(3):CNS43
Lassman AB, Rossi MR, Raizer JJ et al (2005) Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res 11(21):7841–7850
Mellinghoff IK, Wang MY, Vivanco I et al (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 353(19):2012–2024
Schulte A, Liffers K, Kathagen A et al (2013) Erlotinib resistance in EGFR-amplified glioblastoma cells is associated with upregulation of EGFRvIII and PI3Kp110δ. Neuro Oncol 15(10):1289–1301
Jun HJ, Acquaviva J, Chi D et al (2012) Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene 31(25):3039–3050
Ma Y, Tang N, Thompson RC et al (2016) InsR/IGF1R pathway mediates resistance to EGFR inhibitors in glioblastoma. Clin Cancer Res 22(7):1767–1776
Chakravarti A, Loeffler JS, Dyson NJ (2002) Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res 62(1):200–207
He K, Qi Q, Chan CB et al (2013) Blockade of glioma proliferation through allosteric inhibition of JAK2. Sci Signal 6:ra55
de la Iglesia N, Konopka G, Puram SV et al (2008) Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev 22:449–462
Carro MS, Lim WK, Álvarez MJ et al (2010) The transcriptional network for mesenchymal transformation of brain tumours. Nature 463:318–325
Fan QW, Cheng C, Knight ZA et al (2009) EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal 2:ra4
Uht RM, Amos S, Martin PM et al (2007) The protein kinase C-eta isoform induces proliferation in glioblastoma cell lines through an ERK/Elk-1 pathway. Oncogene 26:2885–2893
Aeder SE, Martin PM, Soh JW et al (2004) PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene 23:9062–9069
Cvrljevic AN, Akhavan D, Wu M et al (2011) Activation of Src induces mitochondrial localisation of de2-7EGFR (EGFRvIII) in glioma cells: implications for glucose metabolism. J Cell Sci 124:2938–2950
Struve N, Binder ZA, Stead LF et al (2020) EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene 39(15):3041–3055. https://doi.org/10.1038/s41388-020-1208-5
Eskilsson E, Røsland GV, Solecki G et al (2018) EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro Oncol 20(6):743–752. https://doi.org/10.1093/neuonc/nox191
Weller M, Butowski N, Tran DD et al (2017) Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomized, double-blind, international phase 3 trial. ACT IV trial investigators. Lancet Oncol 18(10):1373–1385
Lassman A, Pugh S, Wang TJ et al (2020) A randomized, double-blind, placebo-controlled phase 3 trial of depatuxizumab mafodotin (ABT-414) in epidermal growth factor receptor (EGFR) amplified (AMP) newly diagnosed glioblastoma (nGBM). Neuro-Oncology 21(Supplement_6):vi17–vi17
Santangelo A, Rossato M, Lombardi G et al (2021) A molecular signature associated with prolonged survival in glioblastoma patients treated with regorafenib. Neuro Oncol 23(2):264–276. https://doi.org/10.1093/neuonc/noaa156
Acknowledgements
The authors are grateful for the generous contribution from the Silberman family and Francisco Castillo (Fundación Neuronas con Corazón) for altruistically promoting the development of brain tumor research in Colombia.
Funding
Supported by Foundation for Clinical and Applied Cancer Research—FICMAC (Bogotá, Colombia) research Grant 014-2018.
Author information
Authors and Affiliations
Contributions
AFC, OA, ARP, EJ, FH, DG, JFR, HC and JAM planned and coordinated the study. DJV, CP, FS, CO, AM, SB, NU, DP, LR, ZLZ, EJ and CS reviewed patient records and composed the database. LR reviewed all histopathology studies. JR, JA, JGR, NS and ARP performed DNA extraction and library preparation and MGMT methylation analysis. AFC, ARP, OA, CR, and RR performed all statistical analysis and data interpretation. AFC, ARP, OA and DJV wrote the initial draft of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
Andrés F. Cardona discloses financial research support from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Foundation Medicine, Roche Diagnostics, Termo Fisher, Broad Institute, Amgen, Flatiron Health, Teva Pharma, Rochem Biocare, Bayer, INQBox and The Foundation for Clinical and Applied Cancer Research—FICMAC. Additionally, he was linked and received honoraria as an advisor, participate in speakers' bureau. He gave expert testimony to EISAI, Merck Serono, Jannsen Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, Guardant Health, Illumina, and Foundation for Clinical and Applied Cancer Research—FICMAC. Oscar Arrieta reports personal fees from Pfizer, grants and individual fees from Astra Zeneca, grants and individual fees from Boehringer-Ingelheim, Lilly, Merck, Bristol Myers Squibb, Roche, outside the submitted work. Christian Rolfo reports relation with Mylan, Archer Biosciences, Oncopass, Inivata, Merck Serono Novartis, MSD, Boehringer Ingelheim, Guardant Health, etc. AstraZeneca as part of Speakers' Bureau. Also, he received research funding from Pfizer and had uncompensated Relationships with OncoDNA, Biomark, and Guardant Health. Leonardo Rojas received honoraria as an advisor, participate in speakers’ bureau from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Astra Zeneca and Eli Lilly. Additionally, he was linked and received honoraria as researcher. All other authors have no conflicts to declare.
Ethical approval
This study was reviewed and approved by ONCOLGroup Platform—Registration No. 2018/21904, Cayre, Clínica del Country, Bogotá, Colombia. All included patients provided signed informed consent and accepted the tumor tissue analysis. An Institutional Review Board and Privacy Board waiver was obtained to facilitate retrospective collection of clinical, pathologic and molecular data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cardona, A.F., Jaramillo-Velásquez, D., Ruiz-Patiño, A. et al. Efficacy of osimertinib plus bevacizumab in glioblastoma patients with simultaneous EGFR amplification and EGFRvIII mutation. J Neurooncol 154, 353–364 (2021). https://doi.org/10.1007/s11060-021-03834-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03834-3