Abstract
Purpose
Given the rarity in the population with adult thalamic gliomas (ATGs), comprehensive characteristics, treatments and survival outcome are not well characterized. This study was conducted to investigate the comprehensive characteristic and treatment of ATGs and identify the prognostic factors associated with overall survival (OS).
Methods
A retrospective analysis of newly diagnosed ATGs who underwent surgical resection consecutively was conducted. Survival analysis of OS was performed by Kaplan–Meier analysis. Cox proportional hazard model was used to investigate the possible prognostic factors associated with OS.
Results
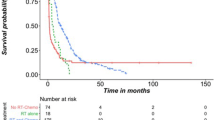
A total of 102 patients with ATG were enrolled in this study. The median age was 41 years (range 18–68 years). There were 56 (54.9%) males. Sixty-two patients (60.8%) had glioblastoma (GBM). Among these patients, 46 patients (45.1%) had GTR/NTR, 50 patients (49.0%) had STR and 6 patients (5.9%) had PR. Postoperatively, 71.6% of these patients received adjuvant therapy. The median OS was 13.6 months (range 1 week–75 months). COX regression analysis revealed that ATG patients with longer duration of symptoms (p = 0.024), better pre-KPS (p = 0.045), maximal resection (p = 0.013), or lower tumor grade (p = 0.002) had longer OS, and these predictors are considered as independent prognostic factors. Survival analysis showed that ATGs with GTR/NTR plus chemoradiotherapy had significant OS advantage compared with other treatment regimens.
Conclusions
This study comprehensively summarized the characteristics, treatments and survival outcomes of ATGs in the largest sample size. Maximal surgical resection can bring survival benefit. Combined-modality therapy regimen of GTR/NTR plus chemoradiotherapy may be better beneficial for OS than other regimens.


Similar content being viewed by others
References
Puget S, Crimmins DW, Garnett MR et al (2007) Thalamic tumors in children: a reappraisal. J Neurosurg 106:354–362. https://doi.org/10.3171/ped.2007.106.5.354
Kramm CM, Butenhoff S, Rausche U et al (2011) Thalamic high-grade gliomas in children: a distinct clinical subset? Neuro-Oncology 13:680–689. https://doi.org/10.1093/neuonc/nor045
Bilginer B, Narin F, Işikay I et al (2014) Thalamic tumors in children. Child’s Nerv Syst 30:1493–1498. https://doi.org/10.1007/s00381-014-2420-9
Steinbok P, Gopalakrishnan CV, Hengel AR et al (2016) Pediatric thalamic tumors in the MRI era: a Canadian perspective. Child’s Nerv Syst 32:269–280. https://doi.org/10.1007/s00381-015-2968-z
Broniscer A, Hwang SN, Chamdine O et al (2018) Bithalamic gliomas may be molecularly distinct from their unilateral high-grade counterparts. Brain Pathol 28:112–120. https://doi.org/10.1111/bpa.12484
Sakka L, Gabrillargues J, Lemaire J-J et al (2010) Anatomy of the human thalamus based on spontaneous contrast and microscopic voxels in high-field magnetic resonance imaging. Neurosurgery 66:161–172. https://doi.org/10.1227/01.neu.0000365617.41061.a3
Yağmurlu K, de Oliveira E, Rhoton A et al (2017) Microsurgical anatomy of the central core of the brain. J Neurosurg. https://doi.org/10.3171/2017.5.jns162897
Moshel YA, Link MJ, Kelly PJ (2007) Stereotactic volumetric resection of thalamic pilocytic astrocytomas. Neurosurgery 61:66–75. https://doi.org/10.1227/01.neu.0000279725.13521.a3
Kelly PJ, Dade Lunsford L, Salcman M (1989) Stereotactic biopsy and resection of thalamic astrocytomas. Neurosurgery 25:185–195. https://doi.org/10.1227/00006123-198908000-00006
McGirr SJ, Kelly PJ, Scheithauer BW (1987) Stereotactic resection of juvenile pilocytic astrocytomas of the thalamus and basal ganglia. Neurosurgery 20:447–452. https://doi.org/10.1227/00006123-198703000-00016
Grigsby PW, Garcia DM, Simpson JR et al (1989) Prognostic factors and results of therapy for adult thalamic and brainstem tumors. Cancer 63:2124–2129. https://doi.org/10.1002/1097-0142(19890601)63:11%3c2124:AID-CNCR2820631109%3e3.0.CO;2-9
McGirt MJ, Woodworth GF, Coon AL et al (2005) Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg 102:897–901. https://doi.org/10.3171/jns.2005.102.5.0897
Baroncini M, Vinchon M, Minéo JF et al (2007) Surgical resection of thalamic tumors in children: approaches and clinical results. Child’s Nerv Syst 23:753–760. https://doi.org/10.1007/s00381-007-0299-4
Sai Kiran NA, Thakar S, Dadlani R et al (2013) Surgical management of thalamic gliomas: case selection, technical considerations, and review of literature. Neurosurg Rev 36:383–392
Cao L, Li C, Zhang Y, Gui S (2015) Surgical resection of unilateral thalamic tumors in adults: approaches and outcomes. BMC Neurol. https://doi.org/10.1186/s12883-015-0487-x
Zhang P, Wang X, Ji N et al (2016) Clinical, radiological, and pathological features of 33 adult unilateral thalamic gliomas. World J Surg Oncol. https://doi.org/10.1186/s12957-016-0820-x
Cinalli G, Aguirre DT, Mirone G et al (2017) Surgical treatment of thalamic tumors in children. J Neurosurg. https://doi.org/10.3171/2017.7.PEDS16463
Rangel-Castilla L, Spetzler RF (2015) The 6 thalamic regions: surgical approaches to thalamic cavernous malformations, operative results, and clinical outcomes. J Neurosurg. https://doi.org/10.3171/2014.11.jns14381
Niu X, Wang T, Yang Y et al (2018) Prognostic factors for the survival outcome of bilateral thalamic glioma: an integrated survival analysis. World Neurosurg 110:e222–e230. https://doi.org/10.1016/j.wneu.2017.10.132
Chi AS, Tarapore RS, Hall MD et al (2019) Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol 145:97–105. https://doi.org/10.1007/s11060-019-03271-3
Jiang H, Yang K, Ren X et al (2019) Diffuse midline glioma with H3 K27M mutation: a comparison integrating the clinical, radiological, and molecular features between adult and pediatric patients. Neuro-Oncology. https://doi.org/10.1093/neuonc/noz152
Albright AL (2004) Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg 100:468–472. https://doi.org/10.3171/ped.2004.100.5.0468
Esquenazi Y, Moussazadeh N, Link TW et al (2018) Thalamic glioblastoma: clinical presentation, management strategies, and outcomes. Neurosurgery 83:76–85. https://doi.org/10.1093/neuros/nyx349
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Wu B, Tang C, Wang Y et al (2018) High-grade thalamic gliomas: microsurgical treatment and prognosis analysis. J Clin Neurosci 49:56–61. https://doi.org/10.1016/j.jocn.2017.12.008
Esquenazi Y, Friedman E, Liu Z et al (2017) The survival advantage of “supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery. 81:275–288
Orringer D, Lau D, Khatri S et al (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 117:851–859. https://doi.org/10.3171/2012.8.JNS12234
Kis D, Máté A, Kincses ZT et al (2014) The role of probabilistic tractography in the surgical treatment of thalamic gliomas. Neurosurgery. https://doi.org/10.1227/NEU.0000000000000333
Zheng X, Xu X, Zhang H et al (2016) A preliminary experience with use of intraoperative magnetic resonance imaging in thalamic glioma surgery: a case series of 38 patients. World Neurosurgery 89:434–441. https://doi.org/10.1016/j.wneu.2016.01.092
Katsevman GA, Turner RC, Urhie O et al (2019) Utility of sodium fluorescein for achieving resection targets in glioblastoma: increased gross- or near-total resections and prolonged survival. J Neurosurg. https://doi.org/10.3171/2018.10.JNS181174
Barzó P, Kincses ZT, Máté A et al (2014) The role of probabilistic tractography in the surgical treatment of thalamic gliomas. Neurosurgery 10:262–272. https://doi.org/10.1227/neu.0000000000000333
Bander ED, Jones SH, Kovanlikaya I, Schwartz TH (2015) Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: a quantitative analysis of FLAIR hyperintensity and apparent diffusion coefficient maps. J Neurosurg 124:1053–1060. https://doi.org/10.3171/2015.4.jns142576
Shooman D, Belli A, Grundy PL (2010) Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J Neurosurg 113:170–178. https://doi.org/10.3171/2009.12.JNS09573
Karam SD, Ney DE, Amini A et al (2016) Combined-modality therapy with radiation and chemotherapy for elderly patients with glioblastoma in the temozolomide era. JAMA Neurol 73:821. https://doi.org/10.1001/jamaneurol.2016.0839
Orr BA, Diaz AK, Zhu X et al (2014) Abstract A15: Variant histone H3 mutations associate with histone modification, DNA methylation, and gene expression changes in pediatric high-grade gliomas. Can Res 73:A15–A15. https://doi.org/10.1158/1538-7445.cec13-a15
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253. https://doi.org/10.1038/ng.1102
Castel D, Philippe C, Calmon R et al (2015) Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130:815–827. https://doi.org/10.1007/s00401-015-1478-0
Kleinschmidt-DeMasters BK, Levy JMM (2018) H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol 37:53–63. https://doi.org/10.5414/NP301085
Karremann M, Gielen GH, Hoffmann M et al (2018) Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro-oncology 20:123–131. https://doi.org/10.1093/neuonc/nox149
Castel D, Philippe C, Kergrohen T et al (2018) Transcriptomic and epigenetic profiling of “diffuse midline gliomas, H3 K27M-mutant” discriminate two subgroups based on the type of histone H3 mutated and not supratentorial or infratentorial location. Acta Neuropathol Commun 6:117. https://doi.org/10.1186/s40478-018-0614-1
Solomon DA, Wood MD, Tihan T et al (2016) Diffuse midline gliomas with histone H3–K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 26:569–580. https://doi.org/10.1111/bpa.12336
Meyronet D, Esteban-Mader M, Bonnet C et al (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro-Oncology 19:1127–1134. https://doi.org/10.1093/neuonc/now274
Wang L, Li Z, Zhang M et al (2018) H3 K27M–mutant diffuse midline gliomas in different anatomical locations. Hum Pathol 78:89–96. https://doi.org/10.1016/j.humpath.2018.04.015
Rao S, Kanuri NN, Nimbalkar V et al (2019) High frequency of H3K27M immunopositivity in adult thalamic glioblastoma. Neuropathology 39:78–84. https://doi.org/10.1111/neup.12537
Louis DN, Perry A, Ellison DW et al (2016) The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Feng J, Hao S, Pan C et al (2015) The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol 46:1626–1632. https://doi.org/10.1016/j.humpath.2015.07.002
Aihara K, Mukasa A, Gotoh K et al (2014) H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro-Oncology 16:140–146. https://doi.org/10.1093/neuonc/not144
Acknowledgements
This study was supported by the Key Research and Development Project of the Department of Science and Technology of Sichuan Province, China (Grant No. 2017SZ0006), Infrastructure Platform from the Department of Science and Technology of Sichuan Province, China (Grant Nos. 2016TJPT0012 and 2015JCPT0002) and Innovation and Sparkle Project of Sichuan University (Grant No. 2082604401004/060).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest related to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niu, X., Wang, T., Zhou, X. et al. Surgical treatment and survival outcome of patients with adult thalamic glioma: a single institution experience of 8 years. J Neurooncol 147, 377–386 (2020). https://doi.org/10.1007/s11060-020-03430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03430-x