Abstract
Purpose
Somatic mutations of the isocitrate dehydrogenase 1 (IDH1) gene, mostly substituting Arg132 with histidine, are associated with better patient survival, but glioma recurrence and progression are nearly inevitable, resulting in disproportionate morbidity and mortality. Our previous studies demonstrated that in contrast to hemizygous IDH1R132H (loss of wild-type allele), heterozygous IDH1R132H is intrinsically glioma suppressive but its suppression of three-dimensional (3D) growth is negated by extracellular glutamate and reducing equivalent. This study sought to understand the importance of 3D culture in IDH1R132H biology and the underlying mechanism of the glutamate effect.
Methods
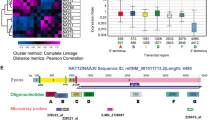
RNA sequencing data of IDH1R132H-heterozygous and IDH1R132H-hemizygous glioma cells cultured under two-dimensional (2D) and 3D conditions were subjected to unsupervised hierarchal clustering and gene set enrichment analysis. IDH1R132H-heterozygous and IDH1R132H-hemizygous tumor growth were compared in subcutaneous and intracranial transplantations. Short-hairpin RNA against glutamate dehydrogenase 2 gene (GLUD2) expression was employed to determine the effects of glutamate and the mutant IDH1 inhibitor AGI-5198 on redox potential in IDH1R132H-heterozygous cells.
Results
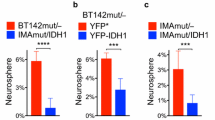
In contrast to IDH1R132H-heterozygous cells, 3D-cultured but not 2D-cultured IDH1R132H-hemizygous cells were clustered with more malignant gliomas, possessed the glioblastoma mesenchymal signature, and exhibited aggressive tumor growth. Although both extracellular glutamate and AGI-5198 stimulated redox potential for 3D growth of IDH1R132H-heterozygous cells, GLUD2 expression was required for glutamate, but not AGI-5198, stimulation.
Conclusion
3D culture is more relevant to IDH1R132H glioma biology. The importance of redox homeostasis in IDH1R132H glioma suggests that metabolic pathway(s) can be explored for therapeutic targeting, whereas IDH1R132H inhibitors may have counterproductive consequences in patient treatment.





Similar content being viewed by others
References
Ostrom QT, Gittleman H, Xu J et al (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 18:v1–v75. https://doi.org/10.1093/neuonc/now207
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. https://doi.org/10.1056/NEJMra0708126
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. https://doi.org/10.1126/science.1164382
Balss J, Meyer J, Mueller W et al (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 116:597–602. https://doi.org/10.1007/s00401-008-0455-2
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Pusch S, Schweizer L, Beck A-C et al (2014) D-2-Hydroxyglutarate producing neo-enzymatic activity inversely correlates with frequency of the type of isocitrate dehydrogenase 1 mutations found in glioma. Acta Neuropathol Commun 2:19. https://doi.org/10.1186/2051-5960-2-19
Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744. https://doi.org/10.1038/nature08617
Xu W, Yang H, Liu Y et al (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30. https://doi.org/10.1016/j.ccr.2010.12.014
Turcan S, Rohle D, Goenka A et al (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483:479–483. https://doi.org/10.1038/nature10866
Lu C, Ward PS, Kapoor GS et al (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483:474–478. https://doi.org/10.1038/nature10860
Huang LE (2019) Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis 11:1299–1307. https://doi.org/10.1093/carcin/bgz134
Piaskowski S, Bienkowski M, Stoczynska-Fidelus E et al (2011) Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br J Cancer 104:968–970. https://doi.org/10.1038/bjc.2011.27
Borodovsky A, Salmasi V, Turcan S et al (2013) 5-azacytidine reduces methylation, promotes differentiation and induces tumor regression in a patient-derived IDH1 mutant glioma xenograft. Oncotarget 4:1737–1747. https://doi.org/10.18632/oncotarget.1408
Luchman HA, Chesnelong C, Cairncross JG, Weiss S (2013) Spontaneous loss of heterozygosity leading to homozygous R132H in a patient-derived IDH1 mutant cell line. Neuro Oncol 15:979–980. https://doi.org/10.1093/neuonc/not064
Chesnelong C, Chaumeil MM, Blough MD et al (2014) Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol 16:686–695. https://doi.org/10.1093/neuonc/not243
Luchman HA, Stechishin OD, Dang NH et al (2012) An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol 14:184–191. https://doi.org/10.1093/neuonc/nor207
Tiburcio PDB, Xiao B, Berg S et al (2018) Functional requirement of a wild-type allele for mutant IDH1 to suppress anchorage-independent growth through redox homeostasis. Acta Neuropathol 135:285–298. https://doi.org/10.1007/s00401-017-1800-0
Tiburcio PDB, Xiao B, Chai Y et al (2018) IDH1R132H is intrinsically tumor-suppressive but functionally attenuated by the glutamate-rich cerebral environment. Oncotarget 9:35100–35113. https://doi.org/10.18632/oncotarget.26203
Sasaki M, Knobbe CB, Itsumi M et al (2012) D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev 26:2038–2049. https://doi.org/10.1101/gad.198200.112
Bardella C, Al-Dalahmah O, Krell D et al (2016) Expression of Idh1R132H in the murine subventricular zone stem cell niche recapitulates features of early gliomagenesis. Cancer Cell 30:578–594. https://doi.org/10.1016/j.ccell.2016.08.017
Pirozzi CJ, Carpenter AB, Waitkus MS et al (2017) Mutant IDH1 disrupts the mouse subventricular zone and alters brain tumor progression. Mol Cancer Res 15:507–520. https://doi.org/10.1158/1541-7786.MCR-16-0485
Amankulor NM, Kim Y, Arora S et al (2017) Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev 31:774–786. https://doi.org/10.1101/gad.294991.116
Waitkus MS, Pirozzi CJ, Moure CJ et al (2018) Adaptive evolution of the GDH2 allosteric domain promotes gliomagenesis by resolving IDH1R132H-induced metabolic liabilities. Cancer Res 78:36–50. https://doi.org/10.1158/0008-5472.CAN-17-1352
Núñez FJ, Mendez FM, Kadiyala P et al (2019) IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaq1427
Chen R, Nishimura MC, Kharbanda S et al (2014) Hominoid-specific enzyme GLUD2 promotes growth of IDH1R132H glioma. Proc Natl Acad Sci USA 111:14217–14222. https://doi.org/10.1073/pnas.1409653111
Khurshed M, Molenaar RJ, Lenting K et al (2017) In silico gene expression analysis reveals glycolysis and acetate anaplerosis in IDH1 wild-type glioma and lactate and glutamate anaplerosis in IDH1-mutated glioma. Oncotarget 8:49165–49177. https://doi.org/10.18632/oncotarget.17106
Lenting K, Khurshed M, Peeters TH et al (2019) Isocitrate dehydrogenase 1-mutated human gliomas depend on lactate and glutamate to alleviate metabolic stress. FASEB J 33:557–571. https://doi.org/10.1096/fj.201800907RR
Choi H, Gillespie DL, Berg S et al (2015) Intermittent induction of HIF-1α produces lasting effects on malignant progression independent of its continued expression. PLoS ONE 10:e0125125. https://doi.org/10.1371/journal.pone.0125125
Rohle D, Popovici-Muller J, Palaskas N et al (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340:626–630. https://doi.org/10.1126/science.1236062
Nix DA, Courdy SJ, Boucher KM (2008) Empirical methods for controlling false positives and estimating confidence in ChIP-Seq peaks. BMC Bioinform 9:523. https://doi.org/10.1186/1471-2105-9-523
Subramanian A, Tamayo P, Mootha VK et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550. https://doi.org/10.1073/pnas.0506580102
Verhaak RGW, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Marin-Valencia I, Yang C, Mashimo T et al (2012) Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab 15:827–837. https://doi.org/10.1016/j.cmet.2012.05.001
Birgersdotter A, Sandberg R, Ernberg I (2005) Gene expression perturbation in vitro–a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 15:405–412. https://doi.org/10.1016/j.semcancer.2005.06.009
Smith SJ, Wilson M, Ward JH et al (2012) Recapitulation of tumor heterogeneity and molecular signatures in a 3D brain cancer model with decreased sensitivity to histone deacetylase inhibition. PLoS ONE 7:e52335. https://doi.org/10.1371/journal.pone.0052335
Noushmehr H, Weisenberger DJ, Diefes K et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. https://doi.org/10.1016/j.ccr.2010.03.017
Bhat KPL, Balasubramaniyan V, Vaillant B et al (2013) Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346. https://doi.org/10.1016/j.ccr.2013.08.001
de Souza CF, Sabedot TS, Malta TM et al (2018) A distinct DNA methylation shift in a subset of glioma CpG island methylator phenotypes during tumor recurrence. Cell Rep 23:637–651. https://doi.org/10.1016/j.celrep.2018.03.107
Tiburcio PDB, Locke MC, Bhaskara S et al Association of gene upregulation with DNA hypomethylation and better outcome in IDH-mutant glioma. J Neurosurg (manuscript in revision)
Jin G, Reitman ZJ, Duncan CG et al (2013) Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Cancer Res 73:496–501. https://doi.org/10.1158/0008-5472.CAN-12-2852
Mazor T, Chesnelong C, Pankov A et al (2017) Clonal expansion and epigenetic reprogramming following deletion or amplification of mutantIDH1. Proc Natl Acad Sci USA 114:10743–10748. https://doi.org/10.1073/pnas.1708914114
Jiang L, Shestov AA, Swain P et al (2016) Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532:255–258. https://doi.org/10.1038/nature17393
Son J, Lyssiotis CA, Ying H et al (2013) Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496:101–105. https://doi.org/10.1038/nature12040
Tateishi K, Wakimoto H, Iafrate AJ et al (2015) Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell 28:773–784. https://doi.org/10.1016/j.ccell.2015.11.006
Molenaar RJ, Botman D, Smits MA et al (2015) Radioprotection of IDH1-mutated cancer cells by the IDH1-mutant inhibitor AGI-5198. Cancer Res 75:4790–4802. https://doi.org/10.1158/0008-5472.CAN-14-3603
Reitman ZJ, Jin G, Karoly ED et al (2011) Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA 108:3270–3275. https://doi.org/10.1073/pnas.1019393108
Turcan S, Fabius AWM, Borodovsky A et al (2013) Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT inhibitor decitabine. Oncotarget 4:1729–1736. https://doi.org/10.18632/oncotarget.1412
Khurshed M, Aarnoudse N, Hulsbos R et al (2018) IDH1-mutant cancer cells are sensitive to cisplatin and an IDH1-mutant inhibitor counteracts this sensitivity. FASEB J 32:6344–6352. https://doi.org/10.1096/fj.201800547R
Andronesi OC, Arrillaga-Romany IC, Ly KI et al (2018) Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun 9:1474–1479. https://doi.org/10.1038/s41467-018-03905-6
Kopinja J, Sevilla RS, Levitan D et al (2017) A brain penetrant mutant IDH1 inhibitor provides In vivo survival benefit. Sci Rep 7:13853. https://doi.org/10.1038/s41598-017-14065-w
Waitkus MS, Diplas BH, Yan H (2018) Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell 34:186–195. https://doi.org/10.1016/j.ccell.2018.04.011
Liu Y, Lu Y, Celiku O et al (2019) Targeting IDH1-mutated malignancies with NRF2 blockade. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djy230
Tang X, Fu X, Liu Y et al (2019) Blockade of glutathione metabolism in IDH1-mutated glioma. Mol Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-19-0103
Huang LE, Cohen AL, Colman H et al (2017) IGFBP2 expression predicts IDH-mutant glioma patient survival. Oncotarget 8:191–202. https://doi.org/10.18632/oncotarget.13329
Acknowledgements
Research reported in this publication utilized the High-Throughput Genomics and Bioinformatic Analysis Shared Resource and the Biorepository and Molecular Pathology Shared Resource at Huntsman Cancer Institute at the University of Utah. This work was supported in part by funds in conjunction with grant P30 CA042014 from the National Cancer Institute of the National Institutes of Health. This work was supported in part by the National Institute of Neurological Disorders and Stroke R21NS108065 Grant. The authors thank Chris Stubben for providing consultation for the analysis of RNA sequencing data, Luming Zhou and Carl T. Wittwer for the assistance in quantitative PCR analysis, Laura Roberts for technical assistance, and Kristin Kraus for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tiburcio, P.D.B., Gillespie, D.L., Jensen, R.L. et al. Extracellular glutamate and IDH1R132H inhibitor promote glioma growth by boosting redox potential. J Neurooncol 146, 427–437 (2020). https://doi.org/10.1007/s11060-019-03359-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03359-w