Abstract
The modified frailty index (mFI) is emerging as a leading measure for preoperative risk assessment using routinely available medical record data. Our objective was to determine if mFI predicts morbidity and mortality in the diverse national cohort of patients undergoing neurosurgery for intracranial neoplasms. We identified patients in the National Surgical Quality Improvement Program who underwent oncologic neurosurgery procedures between 2008 and 2012. The mFI, ranging from 0 to 1, was calculated as the proportion of 11 possible risk factors present. We assessed the associations between mFI and 30-day mortality, neurologic and medical complications, prolonged length of stay, and unfavorable discharge in univariate and multivariable analyses and compare the index to established risk stratification techniques. A total of 9149 patients were identified. Mortality, severe medical complications, prolonged length of stay, and unfavorable discharge increased incrementally with increasing levels of frailty. Severe neurologic complications were highest in those with low frailty. In multivariable logistic regression analysis, increased frailty increased the odds of all adverse outcomes, including neurologic complications. The mFI increased the ability to predict all outcomes beyond available indices and was the most reliable predictor of neurologic complications. The mFI can be calculated from routinely collected medical record data and is predictive of 30-day adverse outcomes in a wide variety of neurosurgical oncology patients. The index may be a useful component of preoperative risk assessment with implications for shared decision-making, perioperative planning, and risk adjusted outcomes measurement in national quality registries.
Similar content being viewed by others
Introduction
As the population ages and neuro-oncologic treatment options expand, older and higher comorbidity patients with brain tumors are increasingly presenting as neurosurgical candidates [1, 2]. Surgical decision-making in this population is complex. In addition to balancing the risks and benefits of operating on a particular tumor, decision-makers must attempt to quantify the added risk that a patient’s underlying health status contributes to the full episode of care. There are countless potential risk factors to consider and medical information may be incomplete. Existing clinical tools tend to be subjective, such as the American Society of Anesthesiology (ASA) class, [1] or narrowly focused on a single outcome, such as cardiac risk assessment [3]. Multivariable predictive models, while useful in clinical research and quality measurement, may be unavailable or impractical for routine clinical use [1, 2, 4]. There is a pressing need for more reliable and transparent tools to quantify preoperative risk.
Based on a combination of functional status and comorbidities, measures of frailty attempt to convey information about a patient’s overall physical function and physiologic reserves [5, 6]. Several studies have demonstrated that the modified frailty index (mFI), a composite score of 11 common comorbidities and function status, can be calculated from routinely collected medical record data and is associated with outcomes in surgical and oncologic populations [7,8,9,10,11,12,13,14]. There is reason to suspect that functional status would play an even larger role in predicting neurooncology outcomes than in other fields. Preoperative neurological deficits are associated with increased risk of adverse outcomes [4, 15] and poor baseline functional status may limit the ability to recover from new neurologic deficits [16]. However, the mFI has not been validated in the oncologic neurosurgery population and its importance relative to other available indices in predicting outcomes remains to be determined.
The objective of our study was to determine whether the mFI, calculated from routinely collected, chart abstracted data in the National Surgical Quality Improvement Program (NSQIP), predicts morbidity and mortality in oncologic neurosurgery. We examined the discriminatory ability of the mFI to predict adverse outcomes in patients undergoing multiple types of surgery for a variety of pathologies and compared predictive ability to available indices in NSQIP.
Methods
Data were obtained from the American College of Surgeons (ACS) NSQIP database [17]. NSQIP captures data from the time of the initial index admission and follows patients for 30 days after surgery. It is a nationwide sample collected from over 400 hospitals. Data are abstracted from the first 40 cases for a given procedure during 8-day sampling cycles and sampling is staggered throughout the year to reduce bias in case selection. Raters are regularly audited and their data excluded if they do not meet preset standards of validity [18].
We identified patients who underwent neurosurgical procedures for intracranial neoplasms between 2008 and 2012. As further shown in Table S1, patients were included if they had (1) a Current Procedural Terminology (CPT) procedure code representing a surgery classified as supratentorial brain (61510, 61545, 61546), supratentorial dural (61512), or infratentorial (61548, 62165); (2) an International Classification of Diseases and Related Conditions, Ninth Revision (ICD-9) diagnosis code representing a pathology classified as primary brain malignant or uncertain (191.x, 192.8, 192.9, 194.3, 194.4, 237.0, 237.1, 237.5, 239.6, 239.7), primary brain benign (225.0, 225.8, 225.9, 227.3, 227.4, 228.00, 228.02), primary dural malignant or uncertain (192.1, 237.6), primary dural benign (213.0, 225.2), primary cranial nerve malignant or benign (192.0, 225.1, 237.72, 237.73), or secondary malignant (198.3, 140.x, 141.x, 142.x, 143.x, 144.x, 145.x, 146.x, 147.x, 148.x, 149.x, 150.x, 151.x, 152.x, 153.x, 154.x, 155.x, 156.x, 156.x, 158.x, 159.x, 160.x, 161.x, 162.x, 163.x, 164.x, 165.x, 170.x, 171.x, 172.x, 173.x, 174.x, 175.x, 176.x, 179.x, 180.x, 181.x, 182.x, 183.x, 184.x, 185.x, 186.x, 187.x, 188.x, 189.x, 190.x, 192.2, 192.3, 193.x, 194.0, 194.1, 194.5, 194.6, 194.8, 194.9, 195.x, 196.x, 197.x, 198.x, 199.x, 200.x, 201.x, 202.x, 203.x, 204.x, 205.x, 206.x, 207.x, 208.x, 209.x, or NSQIP Code for “disseminated cancer”); and (3) were at least 18 years old.
The mFI was calculated using 11 variables from the Canadian Study of Health and Aging (CSHA) Frailty Index [19] that were matched to variables in NSQIP as we have previously described [12] (Table 1). Each variable was scored one point in the numerator for every condition present. The numerator was then divided by the number of variables recorded for that patient (the denominator). Missing data or variables coded as “unknown” were excluded from the calculation of mFI. The mFI thus ranges from 0 to 1 with higher scores representing increasing frailty.
Preoperative demographic and clinical variables included surgery category (supratentorial brain, supratentorial dural, infratentorial, or transsphenoidal), pathology category (primary brain malignant, primary brain benign, primary dural malignant, primary dural benign, primary cranial nerve, and secondary malignant), the risk indices being compared (age, ASA class, and mFI), and other variables not included in the modified frailty index (sex, race, body mass index (BMI), tobacco use, bleeding disorders, hemiplegia, ventilator dependence, sepsis, albumin level, weight loss, transfusion, corticosteroid use, chemotherapy in the past month, radiotherapy in the past 90 days, and emergency status of the case). ASA class is a subjective, overall measure of a patient’s burden of disease prior to surgery. It is coded by the evaluating clinician and is scored from 1, a normal healthy patient, to 6, an organ donor meeting the criteria for brain death [1].
The outcomes measured were 30-day mortality, 30-day severe medical complications, 30-day severe neurologic complications, 30-day any complication, extended length of stay (LOS), and unfavorable disposition. Mortality was defined as death within 30 days of the index procedure. Severe medical complications were defined according to the Clavian class IV categorization, which includes septic shock, cardiac arrest, myocardial infarction, pulmonary embolism, need for more than 48 h of ventilation, or unplanned re-intubation [9, 12, 20]. Coma and cerebrovascular accident or stroke with a new neurologic deficit were categorized as severe neurologic complications. Any complication included the occurrence of a severe medical or neurologic complication as defined above, or a wound complication (wound dehiscence, superficial, deep, or organ space infection), pneumonia, acute renal failure, urinary tract infection, deep vein thrombosis, or sepsis. Prolonged length of stay was defined as hospitalizations lasting longer than 7 days. Unfavorable disposition was defined as a discharge destination other than home or a facility with a higher level of care than before admission.
Distributions of the outcomes across mFI scores were compared using Chi square tests. Multivariable logistic regression models were then constructed for each outcome and model fit statistics were calculated to determine the strength of association between each outcome and mFI, ASA class, age, combined indices, and the full model. The c-statistic is a measure of concordance, or the ability of a model to predict the outcome of interest, and is calculated as the area under a receiver operating characteristic curve (ROC) evaluating true and false positive rates. A value of one corresponds to a perfectly predictive model whereas a value of 0.5 indicates that the model is no more reliable than chance. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical and demographic characteristics of the cohort are presented in Table 2. A total of 9149 patients who underwent resection of intracranial neoplasms were identified from the NSQIP database. Fewer surgeries were performed at increasing levels of frailty. The majority of patients had no frailty (51.5%), while 21.3% had low, 21.5% intermediate, and 5.7% high frailty. Transsphenoidal surgery patients were more likely to have low preoperative frailty (35.1%) than the full cohort (21.3%). Patients with benign primary brain tumors and primary cranial nerve tumors were also less frail. Compared to the full cohort, patients were more likely to have intermediate or high frailty if they had secondary malignant brain tumors, older age, were Black compared to White, or had higher preoperative ASA class, obesity, bleeding disorders, dependent functional status, hemiplegia, ventilator dependence, sepsis, low albumin (< 3.5), weight loss, blood transfusion, corticosteroid use, chemotherapy in the past month, or radiotherapy in the past 90 days.
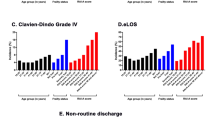
In the univariate analysis (Fig. 1), increased mFI was associated with stepwise increases in the rates of mortality, severe medical complications, prolonged length of stay, and unfavorable discharge. This trend held true in subgroup analyses (see Table S2 in Supplementary Material) of nearly all categories of surgery and pathology with minor exceptions between adjacent levels of frailty and where there were small numbers of cases (i.e. mortality rates among benign tumors and most outcomes for transsphenoidal surgery).
The rate of severe neurologic complications was notably higher (15.8%) in the low frailty group than in those with intermediate or high frailty (5.6 and 12.4%, respectively). This inconsistency contributed to a similar trend in the rate of any complication, which was 24.9% in the low frailty group compared to 18.6% in the intermediate frailty group (although rates of any complication remained highest among the high frailty group at 32.9%).
In the multivariable models (Fig. 2; Table 3), increasing levels of frailty were consistently associated with stepwise increased odds of all adverse outcomes including severe neurologic complications and any complications. Of all adverse outcomes, frailty was most strongly predictive of severe neurologic complications. Compared to patients with no frailty, patients with low frailty (OR 2.005, 95% CI 1.608–2.500), intermediate frailty (OR 3.116, 95% CI 2.304–4.213), and high frailty (OR 5.826, 95% CI 3.983–8.522) were at increased risk of severe neurologic complications. Table S3 represents a sensitivity analysis showing the results of a multivariable model including only those patients for whom all 11 mFI variables were coded (n = 4419).
Frailty as an independent predictor of outcomes in multivariable logistic regression models. Six multivariable models were constructed. Odds ratios reflect the odds of adverse events at each level of frailty with no frailty (mFI = 0) as the reference adjusted for all other predictors in the full models including age, ASA score, surgery category (supratentorial brain, supratentorial dural, infratentorial, or transsphenoidal), pathology category (primary brain malignant, primary brain benign, primary dural malignant, primary dural benign, primary cranial nerve, and secondary malignant), the risk indices being compared (age, ASA class, and mFI level), and other variables not included in the modified frailty index (sex, race, BMI), tobacco use, bleeding disorders, hemiplegia, ventilator dependence, sepsis, albumin level, weight loss, transfusion, corticosteroid use, chemotherapy in the past month, radiotherapy in the past 90 days, and emergency status of the case). *p < 0.05, **p < 0.001, LOS length of stay
Table 3 also demonstrates the comparative predictive ability of frailty, ASA class, and age in the multivariable models of adverse outcomes. Increasing ASA class also reliably predicted stepwise increases of all adverse outcomes. Frailty and ASA class had similar predictive ability for mortality (c-statistics 0.673 vs. 0.704), any complication (0.610 vs. 0.606), prolonged LOS (0.595 vs. 0.620), and unfavorable discharge (0.630 vs. 0.628). Frailty had greater predictive ability than ASA score for severe neurologic complications (0.681 vs. 0.609) whereas it was less predictive of severe medical complications (0.589 vs. 0.629). Both frailty and ASA class had greater predictive ability than age for all models. In fact, after adjustment for other risk factors in the models, only age ≥ 65 was reliably associated with increased odds of most adverse outcomes and the effect sizes (as measured by OR) were relatively small compared to those of ASA class and frailty. All models had improved predictive ability with the combination of frailty, ASA class, and age in the models.
Discussion
Our findings suggest that the modified frailty index, abstracted from routinely collected medical record data, is associated with adverse outcomes in a wide variety of patients undergoing resection of intracranial neoplasms. Incrementally higher levels of frailty independently predicted mortality, severe medical complications, severe neurologic complications, prolonged LOS, and unfavorable discharge. This relationship held true across multiple types of surgery and pathology and regardless of whether or not patients with missing (not coded) variables were included. The index improves the ability to predict adverse outcomes beyond existing preoperative risk indices in NSQIP but predictive ability is greatest when mFI, ASA class, and age are all included.
Surgeons routinely consider and report the surgical morbidity associated with various tumor pathologies, locations, and radiographic features. However, the ability to quantify the added risk that age, functional status, and multiple co-morbidities contribute to a full episode of care remains limited and in practice is often a subjective process [4]. While most population-based studies suggest that advanced age increases postoperative risk, [21] single institution studies demonstrate that some elderly patients can tolerate surgery well [22, 23]. Many comorbidities have been associated with additional perioperative risk [4, 24] but it is not clear which ones are most important in the oncologic neurosurgery population and how best for surgeons to incorporate numerous potential risk factors into their preoperative risk assessment. There is reason to suspect that outcomes in neurosurgical oncology patients may also be more heavily dependent on preoperative functional status than in other surgical populations. Preoperative neurological deficits are associated with increased risk of adverse outcomes [4, 15] and poor baseline functional status may limit the ability to recover from new neurologic deficits [16]. The process of shared decision-making between surgeon and patient would benefit from a simplified tool that can take advantage of routinely collected clinical data to quantify the added risk of co-morbidities and functional status across a wide variety of oncologic neurosurgery patients.
Numerous preoperative risk assessment tools are available, but their usefulness may vary depending on the patient population, the surgical procedure under consideration, and the type of clinical data available [1, 2]. The Goldman Cardiac Risk Index [3] is widely used but is limited to predicting cardiac complications. There are several more comprehensive measures intended for use primarily on admission to an intensive care unit such as the Acute Physiology and Chronic Health Evaluation (APACHE-II), the Physiologic and Severity Score for the Enumeration of Mortality and Morbidity (POSSUM), and the Prognostic Nutritional Index, but these may be too cumbersome for routine preoperative evaluation and are not routinely available in registry data for validation in the neurosurgery population. The Elixhauser and Charlson/Deyo methods were developed to measure comorbidity in administrative claims data [25, 26] and do not easily translate to routine clinical use.
In oncologic neurosurgery practice, the most widely used preoperative risk assessment tools are Karnofsky Performance Score (KPS) and the ASA physical status classification system. KPS is widely used in oncology to classify patients into one of ten levels of functional impairment from disease. However, KPS may be more useful as an outcome measure than in risk assessment, where its reliability has been inconsistent [4, 27,28,29]. It has been criticized as subjective and unidimensional for its emphasis on functional status and failure to specific any particular co-morbidities [30]. It is not available in NSQIP’s data and so comparison was beyond the scope of this study.
The ASA physical status classification system, one of the most widely used preoperative risk assessment tools, is a simple but subjective assessment that classifies patients into one of six levels of overall disease burden [1]. Similar to KPS, ASA is subjective, and does not specify co-morbidities. In our study, frailty and ASA class had similar predictive ability for mortality, any complication, prolonged LOS, and unfavorable discharge. ASA had greater predictive ability for severe medical complications while mFI was more predictive of severe neurologic complications (coma or stroke with new deficit). The combination of ASA class and frailty substantially improved predictive ability for all outcomes, suggesting that both contribute additional information about risk.
The strong association between frailty and severe neurologic complications observed in our study warrants further attention. Prior population level neurosurgical studies have mirrored work in other fields and confirmed the ability of preoperative risk scores to predict postoperative medical complications and mortality, [4, 31, 32] treating neurosurgery patients as any other candidates for surgery. Given the relationship between neurologic deficits and performance status, [4, 15, 16] we anticipated our finding that the mFI, which takes into account functional status, would have greater predictive ability for neurologic outcomes. Frailty predicted neurologic deficits reliably after adjustment for surgery type and pathology category, suggesting it is a useful predictor of neurologic deficit for a wide range of oncologic neurosurgery patients. Of note, in the univariate analyses, low frailty patients were more likely to have severe postoperative neurologic complications than intermediate or high frailty patients. This risk may reflect higher rates of preoperative partial dependence and hemiplegia in the low frailty group (Table 2) compared to the intermediate and high frailty groups, suggesting surgeons were less apt to operate on patients with deficits if they were also medically ill. By mFI definition, patients with no frailty did not have impaired preoperative functional status. There were also notably low rates of preoperative chemotherapy and radiation in the low frailty group, consistent with the notion that these were otherwise healthy patients with preoperative deficits or tumors with high associated risk of deficit in need of surgery without delay for neoadjuvant treatments. These confounders were corrected for in the multivariable model. In this context, the ability to quantify frailty and its associated risks may further assist surgeons in the process of selecting appropriate surgical candidates.
The ability to calculate a simple frailty index in clinical practice and use the same variables in a national registry gives the mFI added value. Surgeons can easily calculate a risk assessment score in the clinical setting and those same variables can later be derived from registry data creating transparency and predictability in risk adjustment outcomes methodology. The persistence of the mFI’s predictive ability in the presence of missing data adds to its practical utility. Findings in NSQIP are likely to be applicable to neurosurgery’s national registry, the Quality Outcomes Database (QOD, formerly N2QOD), [33] in the future. Both registries rely on similar methods of chart abstraction from routinely collected clinical data. The QOD may have the added benefit of capturing additional disease-specific variables relevant to risk prediction in neurosurgery. Ultimately, risk calculators that draw on large national cohorts and factor in numerous variables to provide patient-specific risk assessments will be useful but there remains a need for simple, widely applicable indices for the foreseeable future.
Limitations
We recognize several important limitations to our findings. First, as we have previously discussed, there are several limitations to NSQIP data in general [12]. Although NSQIP ensures that data abstractors are trained and employs methods of data validation, there remains the potential for bias in the record keeping and abstraction processes. While NSQIP captures patients from a large number of hospitals, the centers that participate may not be representative of all hospitals and patients undergoing surgery in the United States. Second, our study was conducted retrospectively, using data not specifically intended or validated for the measurement of frailty. The mFI is unable to measure specific phenotypes of frailty, such as weakness and decreased physical activity that have been captured in prospective studies validating frailty [11]. In NSQIP, abstractors can code a patient’s functional status as independent, partially dependent, totally dependent, or unknown. These and other categorical variables, such as altered sensorium, may be subjective and do not capture more granular information about level of function. Fourth, while we have demonstrated an association between mFI and adverse outcomes in chart abstracted data it is not clear how use of the index would translate in routine clinical practice. Our sensitivity analysis suggests that the mFI predicts adverse outcomes whether or not patients with missing data are included in the cohort, however, the magnitude of the relationships may vary and predictive ability may suffer at the individual level. Measures that rely on the retrospective process of chart abstraction may be more useful for risk adjustment in quality measurement schemes than for Bayesian inference in individual cases of shared decision-making. Prospective validation would be needed. Finally, the scope of our study was limited to the prediction of 30-day adverse events. While this is an improvement over the discharge level data presented in many multi-center database studies, these near-term risks would nonetheless need to be weighed against the potential short and long-term benefits of achieving the surgical aims.
Conclusions
The mFI is a composite score of comorbidity and functional impairment that can be abstracted from routine medical record data. The mFI predicts adverse outcomes in a wide variety of neurosurgical oncology patients. Increased mFI was independently associated with increased mortality, severe medical and neurologic complications, increased LOS, and unfavorable discharge after adjusting for surgery and tumor type, age, ASA class, and other available variables. It improves the ability to predict adverse outcomes beyond age and ASA class and was the most reliable predictor of severe neurologic complications. Predictive ability is greatest when mFI, ASA class, and age, are combined. A frailty score, perhaps in combination with other risk factors, has potential use in preoperative shared decision-making and perioperative management. Frailty can also be calculated from registry data and may therefore have a complementary role in risk adjusted outcomes measurement. Further validation is needed in prospective studies and with variables more specific to neurosurgical oncology patients.
References
Chand M, Armstrong T, Britton G, Nash GF (2007) How and why do we measure surgical risk? J R Soc Med 100:508–512. doi:10.1258/jrsm.100.11.508
Neuman MD, Bosk CL (2012) What we talk about when we talk about risk: refining surgery’s hazards in medical thought. Milbank Q 90:135–159. doi:10.1111/j.1468-0009.2011.00657.x
Goldman L, Caldera DL, Nussbaum SR et al (1977) Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 297:845–850. doi:10.1056/NEJM197710202971601
Reponen E, Tuominen H, Korja M (2014) Evidence for the use of preoperative risk assessment scores in elective cranial neurosurgery. Anesth Analg 119:420–432. doi:10.1213/ANE.0000000000000234
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. The Lancet 381:752–762. doi:10.1016/S0140-6736(12)62167-9
Makary MA, Segev DL, Pronovost PJ et al (2010) Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 210:901–908. doi:10.1016/j.jamcollsurg.2010.01.028
Saxton A, Velanovich V (2011) Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg 253:1223–1229. doi:10.1097/SLA.0b013e318214bce7
Farhat JS, Velanovich V, Falvo AJ et al (2012) Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg 72:1526–1530. doi: 10.1097/TA.0b013e3182542fab (discussion 1530–1531).
Karam J, Tsiouris A, Shepard A et al (2013) Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg 27:904–908. doi:10.1016/j.avsg.2012.09.015
Keller DS, Bankwitz B, Nobel T, Delaney CP (2014) Using frailty to predict who will fail early discharge after laparoscopic colorectal surgery with an established recovery pathway. Dis Colon Rectum 57:337–342. doi:10.1097/01.dcr.0000442661.76345.f5
Adams P, Ghanem T, Stachler R et al (2013) Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg 139:783–789. doi:10.1001/jamaoto.2013.3969
George EM, Burke WM, Hou JY et al (2016) Measurement and validation of frailty as a predictor of outcomes in women undergoing major gynaecological surgery. BJOG 123:455–461. doi:10.1111/1471-0528.13598
Cloney M, D’Amico R, Lebovic J et al (2016) Frailty in geriatric glioblastoma patients: a predictor of operative morbidity and outcome. World Neurosurg 89:362–367. doi:10.1016/j.wneu.2015.12.096
Ali R, Schwalb JM, Nerenz DR et al (2016) Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. J Neurosurg Spine 25(4):1–5. doi: 10.3171/2015.10.SPINE14582
Krivosheya D, Prabhu SS, Weinberg JS, Sawaya R (2016) Technical principles in glioma surgery and preoperative considerations. J Neurooncol. doi:10.1007/s11060-016-2171-4
Sawaya R, Hammoud M, Schoppa D et al (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055. (discussion 1055–6)
ACS National Surgical Quality Improvement Program (2016) In: American College of Surgeons https://www.facs.org/quality-programs/acs-nsqip. Accessed 1 Sept 2016
Shiloach M, Frencher SK Jr, Steeger JE et al (2010) Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 210:6–16. doi:10.1016/j.jamcollsurg.2009.09.031
Rockwood K, Andrew M, Mitnitski A (2007) A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 62:738–743
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. doi:10.1097/01.sla.0000133083.54934.ae
Seicean A, Seicean S, Schiltz NK et al (2013) Short-term outcomes of craniotomy for malignant brain tumors in the elderly. Cancer 119:1058–1064. doi:10.1002/cncr.27851
D’Amico RS, Cloney MB, Sonabend AM et al (2015) The safety of surgery in elderly patients with primary and recurrent glioblastoma. World Neurosurg 84:913–919. doi:10.1016/j.wneu.2015.05.072
Grossman R, Nossek E, Sitt R et al (2013) Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann Surg Oncol 20:1722–1728. doi:10.1245/s10434-012-2748-x
Rolston JD, Han SJ, Lau CY et al (2014) Frequency and predictors of complications in neurological surgery: national trends from 2006 to 2011. J Neurosurg 120:736–745. doi:10.3171/2013.10.JNS122419
Menendez ME, Neuhaus V, van Dijk CN, Ring D (2014) The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res 472:2878–2886. doi:10.1007/s11999-014-3686-7
Boorjian SA, Kim SP, Tollefson MK et al (2013) Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol 190:55–60. doi:10.1016/j.juro.2013.01.010
Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 2:187–193
Crooks V, Waller S, Smith T, Hahn TJ (1991) The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol 46:M139–M144
Reponen E, Korja M, Niemi T et al (2015) Preoperative identification of neurosurgery patients with a high risk of in-hospital complications: a prospective cohort of 418 consecutive elective craniotomy patients. J Neurosurg 123:594–604. doi: 10.3171/2014.11.JNS141970
Salloum RG, Smith TJ, Jensen GA, Lafata JE (2011) Using claims-based measures to predict performance status score in patients with lung cancer. Cancer 117:1038–1048. doi:10.1002/cncr.25677
Barker FG, Curry WT, Carter BS (2005) Surgery for primary supratentorial brain tumors in the United States, 1988 to 2000: the effect of provider caseload and centralization of care. Neuro-oncol 7:49–63. doi:10.1215/S1152851704000146
Grossman R, Mukherjee D, Chang DC et al (2011) Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol 18:521–528. doi:10.1245/s10434-010-1299-2
Asher AL, McCormick PC, Selden NR et al (2013) The National Neurosurgery Quality and Outcomes Database and NeuroPoint Alliance: rationale, development, and implementation. Neurosurg Focus 34:E2. doi: 10.3171/2012.10.FOCUS12311
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Youngerman, B.E., Neugut, A.I., Yang, J. et al. The modified frailty index and 30-day adverse events in oncologic neurosurgery. J Neurooncol 136, 197–206 (2018). https://doi.org/10.1007/s11060-017-2644-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2644-0