Abstract
During the last years, genome wide association studies have discovered common germline genetic variants associated with specific glioma subtypes. We aimed to study the association between these germline risk variants and tumor phenotypes, including copy number aberrations and protein expression. A total of 91 glioma patients were included. Thirteen well known genetic risk variants in TERT, EGFR, CCDC26, CDKN2A, CDKN2B, PHLDB1, TP53, and RTEL1 were selected for investigation of possible correlations with the glioma somatic markers: EGFR amplification, 1p/19q codeletion and protein expression of p53, Ki-67, and mutated IDH1. The CDKN2A/B risk variant, rs4977756, and the CDKN2B risk variant, rs1412829 were inversely associated (p = 0.049 and p = 0.002, respectively) with absence of a mutated IDH1, i.e., the majority of patients homozygous for the risk allele showed no or low expression of mutated IDH1. The RTEL1 risk variant, rs6010620 was associated (p = 0.013) with not having 1p/19q codeletion, i.e., the majority of patients homozygous for the risk allele did not show 1p/19q codeletion. In addition, the EGFR risk variant rs17172430 and the CDKN2B risk variant rs1412829, both showed a trend for association (p = 0.055 and p = 0.051, respectively) with increased EGFR copy number, i.e., the majority of patients homozygote for the risk alleles showed chromosomal gain or amplification of EGFR. Our findings indicate that CDKN2A/B risk genotypes are associated with primary glioblastoma without IDH mutation, and that there is an inverse association between RTEL1 risk genotypes and 1p/19q codeletion, suggesting that these genetic variants have a molecular impact on the genesis of high graded brain tumors. Further experimental studies are needed to delineate the functional mechanism of the association between genotype and somatic genetic aberrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma includes several subtypes. Traditionally, they have been classified solely on histopathological features, though classification is currently changing towards accounting for molecular markers as well [1]. Previous studies have indicated that subtypes of glioma display separate molecular and genetic profiles resulting from their separate etiologic pathways. The somatic mutations and aberrations are sometimes correlated [2], such as the link between IDH1 mutation and 1p/19q codeletion in low grade glioma [3–5]. Some of these markers, like IDH1 mutation and MGMT methylation, have diagnostic value and are useful prognostic and predictive factors relating to patient survival and response to treatment [6–10]. 1p/19q codeletion is thought to be a distinguishing feature for oligodendroglioma and TP53 mutations for astrocytoma, and even though they are not mutually exclusive, they are a clear support in the diagnostic classification [11]. IDH1 mutations are known as an important diagnostic marker, especially for low graded tumors and secondary glioblastoma [12, 13]. In combination with loss of nuclear ATRX expression, IDH1, 1p/19q and TERT promoter mutations define the most frequent type of infiltrative astrocytoma [14, 15], while mutations in the EGFR gene (seen in 35 % of all cases of glioblastoma) are associated with primary glioblastoma [16]. In several of these genes that typically harbor somatic mutations in glioma, genome wide association studies (GWAS) have discovered common germline variants that are associated with risk of developing glioma, including variants in EGFR, CDKN2A, TERT, and TP53 [17–22]. Furthermore, germline variants at 8q24.21 are known to be associated with oligodendroglial tumors and astrocytoma with mutated IDH1 or IDH2 [23]. Several single nucleotide polymorphisms (SNPs) have also been shown to associate with tumor grade. Variants in CDKN2B and RTEL1 are strongly associated with high-grade glioma while variants in CCDC26 and PHLDB1 are associated with low-grade glioma [18, 24].
To investigate whether germline genetic risk variants are linked to specific molecular characteristics of the tumor, we selected 13 glioma risk variants established in the previous studies, mainly GWAS (Supplementary Table 1), and studied their correlation with the glioma somatic biomarkers: EGFR alteration, 1p/19q codeletion, IDH1 mutation, p53 and Ki67 protein expression. We used immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) analyses to assess the biomarkers. In addition, FISH results were compared with the results from one of our previous studies, where somatic copy number data were calculated from SNP array [25] profiles, to explore if the different methods can detect similar genetic aberrations.
Materials and methods
Study population and tumor specimens
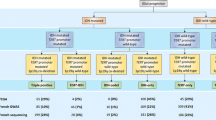
Paraffin-embedded glioma tissues were available from 91 patients for the present study, and the sample set and its characteristics are listed in Table 1. Histologically, 33 of the tumors were grade II-III glioma and 58 were glioblastoma (Table 1). The patients in the present study overlap with the ones included in a paper by Wibom et al. [25], where the ASCAT algorithm [26] was employed to calculate somatic genome-wide allele-specific copy number profiles (i.e., ASCAT profiles). The overlap is constituted by 59 patients that were included in both studies (Table 1; Supplementary Table 2). Informed consent was obtained from all individual participants included in the study. The ethical board approval was obtained for all experiments, in accordance with the Umeå University guidelines.
Immunohistochemistry (IHC)
A neuropathologist identified histologically representative tumor regions that were stained by hematoxylin and eosin. Tissue sections were cut at 4 µm and the IHC was performed using the Ventana Benchmark system (Ventana Medical System, Tucson, AZ, USA). As a pre-treatment step, tissues were subjected to heat-induced epitope retrieval with the Cell Conditioning 2 solution (Ventana, Tucson, AZ, USA), 24 min for Ki-67 (30-9) (Ventana, Tucson, AZ, USA), 32 min for p53 (DO-7) (Ventana, Tucson, AZ, USA) and IDH1 (R132H) (Dianova, Hamburg, Germany). The antibody concentrations were 2 µg/ml for Ki-67, 184 µg/ml for p53, and 4 µg/ml for IDH1. Two independent observers evaluated the stained slides. Proliferation index was evaluated using Ki-67 antibody staining and calculated by determining the percentage of immunopositive nuclei. A total of 100-500 nuclei were counted. The tumors were divided into two groups, less aggressive (<15 %) and more aggressive ≥15 %). The consensus for p53 was scored in four different categories: no immunoreactivity (0 %), faint (≤50 %), moderate (50–75 %), and strong (≥75 %) immunoreactivity. IDH1 was scored in two categories: (0–10 %) for negative immunoreactivity, and (≥10 %) for positive immunoreactivity.
Fluorescence in situ hybridization (FISH)
Tissue sections for 1p, 19q, and EGFR FISH staining were cut at 4 µm. The slides were deparaffinized, dehydrated, and placed in pretreatment solution (Vysis, Illinois, USA) followed by rinse in purified H2O and 2 × SSC. The slides were then treated for 45 min in 50 ml of solution (NaCl pH 2.0) containing 25 mg protease (Vysis, Illinois, USA), and rinsed in H2O and 2 × SSC. Locus-specific probes for EGFR (7p12), 1p36/1q13 and 19p13/19q13 were used as recommended by the manufacturer (Vysis, Illinois, USA). In short, probes were applied and a coverslip was placed over the target area, followed by sealing with rubber cement to prevent evaporation of the probe. Simultaneous denaturation of the probe and target was carried out on the THERMOBrite (Abbott Molecular, Illinois, USA) at 74 °C for 6 min. Hybridization was performed by placing the slides in a humidified chamber at 37 °C for overnight incubation. After hybridization, slides were treated in a post-hybridization wash of 2 × SSC solution containing 0.3 % NP40 at 73 °C and nuclei were counterstained by DAPI (Sigma-Aldrich, USA) nuclear counterstain. Antifade (CitiFluor, London, UK) was applied and the sections were viewed using a Zeiss Axio Imager Z1fluorescent microscope with a dual green/orange filter (Vysis, Illinois, USA). Three observers evaluated the slides and the evaluation was based on 100 intact non-overlapping nuclei that were counted for both the green and orange signals. The ratio of EGFR was calculated using the criteria developed in previous studies [27–29]. A ratio between the locus specific probe (EGFR) and the control probe CEP7 (EGFR/CEP7) was calculated where ratios equal to 1 was considered as normal, while more than 10 % cells with a ratio between 1 and 2 was considered as chromosomal gain and more than 10 % cells with a ratio greater than 2 was considered as amplification. The ratio between the locus specific probe and control probe for both 1p (1p36/1q25) and 19q (19q13/19p13) was calculated using the criteria used in the clinical routine practice [30], 1p36/1q25 ratios < 0.88 and 19q13/19p13 ratios < 0.74 in more than 12 % of the cells were considered as deleted.
SNP array
Data was taken from our previous study [25] where DNA was extracted from glioma tissue using QIAmp Mini Kit (QIAGEN GmbH, Hilden, Germany) and genotyped using Illumina HumanOmni1-Quad BeadChips. The ASCAT algorithm [26] (version 2.0) was used to calculate somatic whole-genome allele-specific copy number profiles (ASCAT-profiles), as well as estimates of tumor cell content and tumor cell ploidy. For comparison between FISH and ASCAT, we extracted the median total copy number from the ASCAT profiles for the genomic regions corresponding to the FISH probes. These copy number data were subsequently used to mimic the sample classification based on FISH data, by calculating the same ratios and using the same cutoff values that had been used for classification by FISH. More details about the SNPs can be found in supplementary Table 1 and samples included in analyses with both FISH and ASCAT are shown in Table 1.
Statistical analyses
The associations between the biomarkers and genetic risk variants as well as comparisons of different methods were evaluated using the χ 2 test or the Fisher’s exact test. The significance level was set at p < 0.05. Six genetic variants (rs2252586, rs17172430, rs11979158, rs4295627, rs55705857, and rs78378222) were not genotyped by the SNP array. Therefore, these variants were imputed using the software IMPUTE2 with data from the 1000 Genomes Project as the reference population. One SNP, rs55705857 was excluded from further analysis since it could not be imputed with high certainty (imputation score < 0.80) (Supplementary Table 1).
Results
Eighty glioma patients were successfully analyzed for EGFR copy number variation and 1p/19q codeletion, however two samples were excluded since the ratio was below 1 and there were too few patients to make a separate group for these two samples. EGFR amplification was observed in 24 of 78 (30.8 %) glioma tumors and in 18 of 47 (38.3 %) glioblastoma tumors. 1p/19q codeletion was observed in 14 of 78 (17.9 %) glioma tumors and 8 of 50 (16.0 %) glioblastoma tumors. Due to lack of patient material and failed analyses different numbers of glioblastoma tumors are analyzed for EGFR amplification and 1p/19q codeletion (Table 2).
The blood samples corresponding to the tumor samples were analyzed with the SNP array. Four genetic risk variants showed association with the investigated glioma biomarkers (Table 3). The CDKN2A/B risk variant (rs4977756) and the CDKN2B risk variant (rs1412829) were both inversely associated with expression of mutated IDH1 (p = 0.049 and p = 0.002, respectively) since for both these variants, the majority of patients homozygous for the risk allele (G) showed no or low (0–10 % immunoreactivity) expression of mutated IDH1. The CDKN2B risk variant, rs1412829 and the CDKN2A/B risk variant, rs4977756 are both located on chromosome 9p21 within the same gene cluster as the non-coding RNA CDKN2B-AS1 (also known as ANRIL), and these risk variants are largely dependent of each other in terms of linkage disequilibrium (LD) since they are both located within the same haplotype block (r2 = 0.741; D′ = 0.888). The RTEL1 risk variant (rs6010620) was inversely associated with 1p/19q codeletion (p = 0.013) since the majority of patients homozygous for the risk allele (G) showed no 1p/19q codeletion. In addition, we observed a trend of higher frequency of EGFR amplified tumors in patients homozygous for the EGFR risk variant (rs17172430) and the CDKN2B risk variant (rs1412829). This finding was however not statistically significant. None of the other evaluated risk variants showed any significant associations with the investigated glioma biomarkers.
To compare the copy number profiles achieved by applying ASCAT to SNP array data with results from the FISH analysis, we focused on 1p/19q codeletion and EGFR amplification, because these features have clinical implications. For 1p/19q codeletion, there were 55 patients with data from both methods available, and 59 patients with data from both methods were available for EGFR amplification. The comparison yielded entirely disparate results with regards to 1p/19q codeletion, where FISH detected 14 samples displaying this aberration whereas none was detected based on SNP array data (Supplementary Table 3). The similarity in results from the two techniques was greater with regards to EGFR amplification. Using FISH, we detected 24 samples with EGFR amplification, of these 23 had ASCAT profiles available and 17 of them displayed EGFR amplification also by the SNP array approach (Table 4). In addition, 3 samples displayed chromosomal gain in EGFR as analyzed by FISH, of these 2 had ASCAT profiles available but none of them displayed chromosomal gain in EGFR also by the SNP array approach (Table 4).
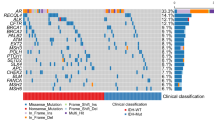
Based on proliferation index, 46 of 91 glioma tumors were considered less aggressive and 45 of 91 were more aggressive. Expression of mutated IDH1 was found in 15 of 90 glioma tumors, whereas 4 of 57 cases in the glioblastoma subgroup were positive for mutated IDH1. Almost all glioma patients, 85 of 89, showed p53 expression. In the glioblastoma subgroup, 38 of 56 showed faint to moderate protein expression while 17 patients demonstrated strong p53 protein expression (Fig. 1). Due to lack of patient material and failed analyses different numbers of samples are analyzed for the different biomarkers.
Discussion
There are specific molecular markers in glioma characterization used to define the histological subtypes and grades of malignancy, as well as markers of diagnostic and prognostic value, and markers that may be used to predict response to treatment. Exploring an association between germline genetic variation and molecular alterations could be a key for definition of unique molecular based subtypes of glioma.
Previous studies have observed that some genetic variants are associated with tumor grade, like risk variants in the CDKN2B, RTEL1, and TERT regions [18, 31], which show association with high grade glioma, while risk variants in the CCDC26 and PHLDB1 regions are associated with low grade glioma involving IDH mutation, and 1p/19q codeletion [17, 31]. Although, association with tumor grade was not analyzed in our study due to the small number of low grade glioma, we found two risk variants in the CDKN2A and CDKN2B regions associated with mutated IDH1 (Table 3). The risk variant near CDKN2B (rs1412829) is the same risk variant associated with tumor grade in the study by Wrensch et al. [18]. We found expression of mutated IDH1 in few glioblastoma cases, which is in concordance with previous studies [4]. These findings might have clinical implications as a potential predictive marker, since recently updated data from the RTOG 9402 trial showed that IDH mutations predict the benefit of adjuvant chemotherapy in grade III glioma [32]. Other studies have shown that oligodendroglial tumors and glioma with mutated IDH1 are strongly associated with the chromosome 8q24.21 risk variant (rs55705857) [23]. Conversely, and probably due to low statistical power in our study, we do not see any strong association between IDH1 mutations and the chromosome 8q24.21 risk variant. One risk variant in RTEL1 (rs6010620) that previously has shown association with 1p/19q codeletion [31], was significantly associated with 1p/19q codeletion also in our study. It has earlier been shown that genetic variants within or near the RTEL1 (20q13) regions are strongly associated with glioblastoma [33]. RTEL1 has been hypothesized to be involved in the resolution of D loops that occur during homologous recombination, and is together with TERT supposed to play a role in regulating telomere length [34, 35]. We found an inverse association between 1p/19q codeletion and the risk variant in RTEL1 (rs6010620) but not the risk variant in TERT (rs2736100). Although the number of patients homozygous for the non-risk genotype in this comparison was only 4, our results are in line with previous studies, and suggest that germline glioma risk variants might be involved in the development and progression of high grade glioma. Nevertheless, since the majority of the genetic variants analyzed in this study are located in introns or intergenic regions, and do not result in amino acid changes in transcribed proteins, the mechanism of action behind these associations need to be further elucidated.
We have previously shown that two risk variants (rs17172430 and rs11979158) in EGFR are associated with homozygous deletion at the CDKN2A/B locus, and that one of the risk variants (rs17172430) in EGFR also shows association with allele specific loss of heterozygosity at the EGFR locus [25]. In this study, both the EGFR risk variant (rs17172430) and the CDKN2B risk variant (rs1412829) showed a trend for an association with chromosomal gain and amplification in EGFR. Similar trends were observed in the same sample set based on ASCAT copy number profiles, but they did not validate when tested on a TCGA data set in our previous study [25]. The association with chromosomal gain might indicate that these genotypes are associated with increased genetic instability where the tumor is more prone to have genetic aberrations with loss of one allele and copy number increase of the remaining allele. The genetic variants in EGFR that have been associated with glioma risk are not closely linked in the genome, and therefore these genotypes could give disparate result. In this study, the sample number is relatively small and thus suffering from limited statistical power to detect associations, particularly affecting low-frequency variants and variants with small effect size. The genotype-phenotype associations are not significant following adjustment to the family-wise error rate (Bonferroni correction). However, this procedure to adjust for multiple testing might be too stringent given that some investigated variables in this study are not independent. Larger glioma studies with dense tagging of the EGFR gene are required to elucidate the number of true associated genetic variants.
In addition, we have compared the present study with a previous study, where ASCAT profiles were calculated on a set of samples that overlapped with the samples included in this study. We observed that the different methodologies identifies dissimilar types of genetic aberrations. The SNP array approach cover the whole genome but might be considered less sensitive than FISH to detect aberrations in tumor subclones. For 1p/19q codeletion, the aberrations that the FISH analysis detected was not identified by the ASCAT analysis (data not shown), while for EGFR, results from the two methods showed a better correlation (Table 4). Both methods compared in this study have advantages and disadvantages. Establishment of a good threshold level for positive results is important for avoiding over interpretation of small cell populations when using FISH analysis and SNP array. However, the threshold for 1p/19q codeletion is well established in the clinic [30] and the threshold of EGFR amplification is well studied [27–29]. The FISH analysis technique uses fluorescently labeled DNA probes to detect chromosomal abnormalities. Applying ASCAT to SNP array data allow us to estimate both tumor cell content and tumor cell ploidy, which cannot be detected by FISH analysis. A uniparental disomy, when cancer cells have lost one chromosome in the presence of duplication of the other chromosomal allele, cannot be detected by FISH analysis, while this can be detected by ASCAT. FISH analysis with locus-specific probe does not allow testing for multiple chromosomal loci which can be detected by SNP arrays. On the other hand, the ASCAT algorithm assumes that the tumor sample is from the same clone and will ignore the heterogeneity of the tumor, which is a well-known aspect of glioma and this could be an explanation why ASCAT fails to detect 1p/19q codeletion.
In conclusion, even though the results need to be taken with caution since this study represents a small sample size, we found inverse associations between genetic risk variants in CDKN2A/2B, RTEL1 IDH1 mutation and 1p/19q codeletion, in line with previous studies. Whereas the results revealing that risk variants in EGFR and CDKN2B both showed a trend for association with EGFR copy number variation are new findings. The idea that the genetic variants could be used as a complementary diagnostic approach for tumors difficult to assess for conclusive biopsies is an interesting diagnostic concept in glioma, where there seem to be a limited number of genetic predisposing loci and robust biomarkers that might be added to diagnostics.
References
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. doi:10.1038/nature07385
Ohgaki H, Kleihues P (2009) Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci 100:2235–2241. doi:10.1111/j.1349-7006.2009.01308.x
Labussiere M, Idbaih A, Wang XW, Marie Y, Boisselier B, Falet C, Paris S, Laffaire J, Carpentier C, Criniere E, Ducray F, El Hallani S, Mokhtari K, Hoang-Xuan K, Delattre JY, Sanson M (2010) All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology 74:1886–1890. doi:10.1212/WNL.0b013e3181e1cf3a
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27:4150–4154. doi:10.1200/JCO.2009.21.9832
Zou P, Xu H, Chen P, Yan Q, Zhao L, Zhao P, Gu A (2013) IDH1/IDH2 mutations define the prognosis and molecular profiles of patients with gliomas: a meta-analysis. PLoS One 8:e68782. doi:10.1371/journal.pone.0068782
Nikiforova MN, Hamilton RL (2011) Molecular diagnostics of gliomas. Arch Pathol Lab Med 135:558–568. doi:10.1043/2010-0649-RAIR.1
Ducray F, El Hallani S, Idbaih A (2009) Diagnostic and prognostic markers in gliomas. Curr Opin Oncol 21:537–542. doi:10.1097/CCO.0b013e32833065a7
Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G (2010) Molecular diagnostics of gliomas: state of the art. Acta Neuropathol 120:567–584. doi:10.1007/s00401-010-0736-4
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75:1560–1566. doi:10.1212/WNL.0b013e3181f96282
Idbaih A, Marie Y, Lucchesi C, Pierron G, Manie E, Raynal V, Mosseri V, Hoang-Xuan K, Kujas M, Brito I, Mokhtari K, Sanson M, Barillot E, Aurias A, Delattre JY, Delattre O (2008) BAC array CGH distinguishes mutually exclusive alterations that define clinicogenetic subtypes of gliomas. Int J Cancer 122:1778–1786. doi:10.1002/ijc.23270
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 116:597–602. doi:10.1007/s00401-008-0455-2
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V, Mittelbronn M, Schittenhelm J, Herold-Mende C, Unterberg A, Platten M, Weller M, Wick W, Pfister SM, von Deimling A (2015) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol 129:133–146. doi:10.1007/s00401-014-1370-3
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O’Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB (2015) Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med 372:2499–2508. doi:10.1056/NEJMoa1407279
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, Simon M, Marie Y, Boisselier B, Delattre JY, Hoang-Xuan K, El Hallani S, Idbaih A, Zelenika D, Andersson U, Henriksson R, Bergenheim AT, Feychting M, Lonn S, Ahlbom A, Schramm J, Linnebank M, Hemminki K, Kumar R, Hepworth SJ, Price A, Armstrong G, Liu Y, Gu X, Yu R, Lau C, Schoemaker M, Muir K, Swerdlow A, Lathrop M, Bondy M, Houlston RS (2009) Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41:899–904. doi:10.1038/ng.407
Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, Ballman KV, Berger M, Buckner JC, Chang S, Giannini C, Halder C, Kollmeyer TM, Kosel ML, LaChance DH, McCoy L, O’Neill BP, Patoka J, Pico AR, Prados M, Quesenberry C, Rice T, Rynearson AL, Smirnov I, Tihan T, Wiemels J, Yang P, Wiencke JK (2009) Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet 41:905–908. doi:10.1038/ng.408
Stacey SN, Sulem P, Jonasdottir A, Masson G, Gudmundsson J, Gudbjartsson DF, Magnusson OT, Gudjonsson SA, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Nexo BA, Tjonneland A, Overvad K, Rudnai P, Gurzau E, Koppova K, Hemminki K, Corredera C, Fuentelsaz V, Grasa P, Navarrete S, Fuertes F, Garcia-Prats MD, Sanambrosio E, Panadero A, De Juan A, Garcia A, Rivera F, Planelles D, Soriano V, Requena C, Aben KK, van Rossum MM, Cremers RG, van Oort IM, van Spronsen DJ, Schalken JA, Peters WH, Helfand BT, Donovan JL, Hamdy FC, Badescu D, Codreanu O, Jinga M, Csiki IE, Constantinescu V, Badea P, Mates IN, Dinu DE, Constantin A, Mates D, Kristjansdottir S, Agnarsson BA, Jonsson E, Barkardottir RB, Einarsson GV, Sigurdsson F, Moller PH, Stefansson T, Valdimarsson T, Johannsson OT, Sigurdsson H, Jonsson T, Jonasson JG, Tryggvadottir L, Rice T, Hansen HM, Xiao Y, Lachance DH, O’Neill BP, Kosel ML, Decker PA, Thorleifsson G, Johannsdottir H, Helgadottir HT, Sigurdsson A, Steinthorsdottir V, Lindblom A, Sandler RS, Keku TO, Banasik K, Jorgensen T, Witte DR, Hansen T, Pedersen O, Jinga V, Neal DE, Catalona WJ, Wrensch M, Wiencke J, Jenkins RB, Nagore E, Vogel U, Kiemeney LA, Kumar R, Mayordomo JI, Olafsson JH, Kong A, Thorsteinsdottir U, Rafnar T, Stefansson K (2011) A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet 43:1098–1103. doi:10.1038/ng.926
Schwartzbaum JA, Xiao Y, Liu Y, Tsavachidis S, Berger MS, Bondy ML, Chang JS, Chang SM, Decker PA, Ding B, Hepworth SJ, Houlston RS, Hosking FJ, Jenkins RB, Kosel ML, McCoy LS, McKinney PA, Muir K, Patoka JS, Prados M, Rice T, Robertson LB, Schoemaker MJ, Shete S, Swerdlow AJ, Wiemels JL, Wiencke JK, Yang P, Wrensch MR (2010) Inherited variation in immune genes and pathways and glioblastoma risk. Carcinogenesis 31:1770–1777. doi:10.1093/carcin/bgq152
Andersson U, Schwartzbaum J, Wiklund F, Sjostrom S, Liu Y, Tsavachidis S, Ahlbom A, Auvinen A, Collatz-Laier H, Feychting M, Johansen C, Kiuru A, Lonn S, Schoemaker MJ, Swerdlow AJ, Henriksson R, Bondy M, Melin B (2010) A comprehensive study of the association between the EGFR and ERBB2 genes and glioma risk. Acta Oncol 49:767–775. doi:10.3109/0284186X.2010.480980
Sanson M, Hosking FJ, Shete S, Zelenika D, Dobbins SE, Ma Y, Enciso-Mora V, Idbaih A, Delattre JY, Hoang-Xuan K, Marie Y, Boisselier B, Carpentier C, Wang XW, Di Stefano AL, Labussiere M, Gousias K, Schramm J, Boland A, Lechner D, Gut I, Armstrong G, Liu Y, Yu R, Lau C, Di Bernardo MC, Robertson LB, Muir K, Hepworth S, Swerdlow A, Schoemaker MJ, Wichmann HE, Muller M, Schreiber S, Franke A, Moebus S, Eisele L, Forsti A, Hemminki K, Lathrop M, Bondy M, Houlston RS, Simon M (2011) Chromosome 7p11.2 (EGFR) variation influences glioma risk. Hum Mol Genet 20:2897–2904. doi:10.1093/hmg/ddr192
Jenkins RB, Xiao Y, Sicotte H, Decker PA, Kollmeyer TM, Hansen HM, Kosel ML, Zheng S, Walsh KM, Rice T, Bracci P, McCoy LS, Smirnov I, Patoka JS, Hsuang G, Wiemels JL, Tihan T, Pico AR, Prados MD, Chang SM, Berger MS, Caron AA, Fink SR, Halder C, Rynearson AL, Fridley BL, Buckner JC, O’Neill BP, Giannini C, Lachance DH, Wiencke JK, Eckel-Passow JE, Wrensch MR (2012) A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet 44:1122–1125. doi:10.1038/ng.2388
Simon M, Hosking FJ, Marie Y, Gousias K, Boisselier B, Carpentier C, Schramm J, Mokhtari K, Hoang-Xuan K, Idbaih A, Delattre JY, Lathrop M, Robertson LB, Houlston RS, Sanson M (2010) Genetic risk profiles identify different molecular etiologies for glioma. Clin Cancer Res 16:5252–5259. doi:10.1158/1078-0432.CCR-10-1502
Wibom C, Ghasimi S, Van Loo P, Brannstrom T, Trygg J, Lau C, Henriksson R, Bergenheim T, Andersson U, Ryden P, Melin B (2012) EGFR gene variants are associated with specific somatic aberrations in glioma. PLoS One 7:e47929. doi:10.1371/journal.pone.0047929
Van Loo P, Nordgard SH, Lingjaerde OC, Russnes HG, Rye IH, Sun W, Weigman VJ, Marynen P, Zetterberg A, Naume B, Perou CM, Borresen-Dale AL, Kristensen VN (2010) Allele-specific copy number analysis of tumors. Proc Natl Acad Sci USA 107:16910–16915. doi:10.1073/pnas.1009843107
Dacic S, Flanagan M, Cieply K, Ramalingam S, Luketich J, Belani C, Yousem SA (2006) Significance of EGFR protein expression and gene amplification in non-small cell lung carcinoma. Am J Clin Pathol 125:860–865. doi:10.1309/H5UW-6CPC-WWC9-2241
Layfield LJ, Willmore C, Tripp S, Jones C, Jensen RL (2006) Epidermal growth factor receptor gene amplification and protein expression in glioblastoma multiforme: prognostic significance and relationship to other prognostic factors. Appl Immunohistochem Mol Morphol 14:91–96. doi:10.1097/01.pai.0000159772.73775.2e
Lopez-Gines C, Gil-Benso R, Ferrer-Luna R, Benito R, Serna E, Gonzalez-Darder J, Quilis V, Monleon D, Celda B, Cerda-Nicolas M (2010) New pattern of EGFR amplification in glioblastoma and the relationship of gene copy number with gene expression profile. Mod Pathol 23:856–865. doi:10.1038/modpathol.2010.62
Burger PC, Minn AY, Smith JS, Borell TJ, Jedlicka AE, Huntley BK, Goldthwaite PT, Jenkins RB, Feuerstein BG (2001) Losses of chromosomal arms 1p and 19q in the diagnosis of oligodendroglioma. A study of paraffin-embedded sections. Mod Pathol 14:842–853. doi:10.1038/modpathol.3880400
Di Stefano AL, Enciso-Mora V, Marie Y, Desestret V, Labussiere M, Boisselier B, Mokhtari K, Idbaih A, Hoang-Xuan K, Delattre JY, Houlston RS, Sanson M (2013) Association between glioma susceptibility loci and tumour pathology defines specific molecular etiologies. Neuro Oncol 15:542–547. doi:10.1093/neuonc/nos284
Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, Buckner JC, Fink KL, Souhami L, Laperriere NJ, Huse JT, Mehta MP, Curran WJ Jr (2014) Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 32:783–790. doi:10.1200/JCO.2013.49.3726
Jenkins RB, Wrensch MR, Johnson D, Fridley BL, Decker PA, Xiao Y, Kollmeyer TM, Rynearson AL, Fink S, Rice T, McCoy LS, Halder C, Kosel ML, Giannini C, Tihan T, O’Neill BP, Lachance DH, Yang P, Wiemels J, Wiencke JK (2011) Distinct germ line polymorphisms underlie glioma morphologic heterogeneity. Cancer Genet 204:13–18. doi:10.1016/j.cancergencyto.2010.10.002
Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O’Neil NJ, Petalcorin MI, Martin JS, Collis SJ, Cantor SB, Auclair M, Tissenbaum H, West SC, Rose AM, Boulton SJ (2008) RTEL1 maintains genomic stability by suppressing homologous recombination. Cell 135:261–271. doi:10.1016/j.cell.2008.08.016
Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T (2009) Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138:90–103. doi:10.1016/j.cell.2009.06.021
Acknowledgments
This study was supported by Umeå Hospital Cutting Edge Grant, Swedish Cancer Foundation, Swedish Research Council and Acta Oncologica Foundation through Swedish Royal Academy of Science. We thank Ulla-Stina Spetz for performing the IHC staining and Jonas Sörlin, Charlotte Andersson, and Birgitta Hagström for the evaluation of the FISH analyses. This research was conducted using the resources of High Performance Computing Center North (HPC2N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ghasimi, S., Wibom, C., Dahlin, A.M. et al. Genetic risk variants in the CDKN2A/B, RTEL1 and EGFR genes are associated with somatic biomarkers in glioma. J Neurooncol 127, 483–492 (2016). https://doi.org/10.1007/s11060-016-2066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2066-4